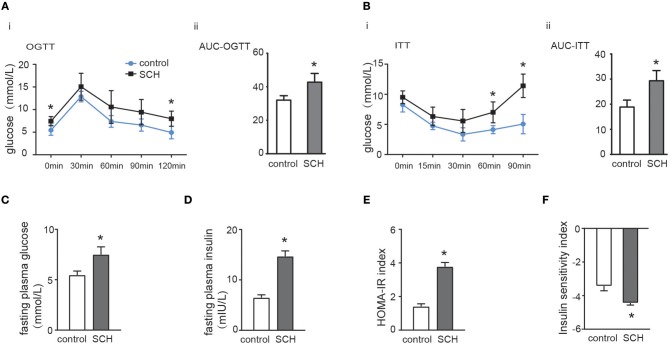
Figure 2.
SCH mice exhibited abnormal glucose metabolism and IR. Male C57/BL6 mice were given methimazole (MMI, 0.08 mg/kg·BW·d, SCH group) or a corresponding volume of vehicle (control group). (A) Oral glucose tolerance test (OGTT) was implemented to detect glucose tolerance at the 13th week of administering MMI to mice in the drinking water. (B) Insulin tolerance test (ITT) was implemented to assess insulin-stimulated glucose disposal at the 14th week of administering MMI to mice in the drinking water. (C) The fasting plasma glucose level was assayed at the 14th week (n = 4–6). (D) The fasting plasma insulin level was assayed at the 14th week (n = 4–6). (E) Homeostasis Model Assessment-Insulin Resistance (HOMA-IR) index was calculated. (F) Insulin sensitivity index (ISI) was calculated. The data are presented as the mean ± SD. *p < 0.05 compared with control.