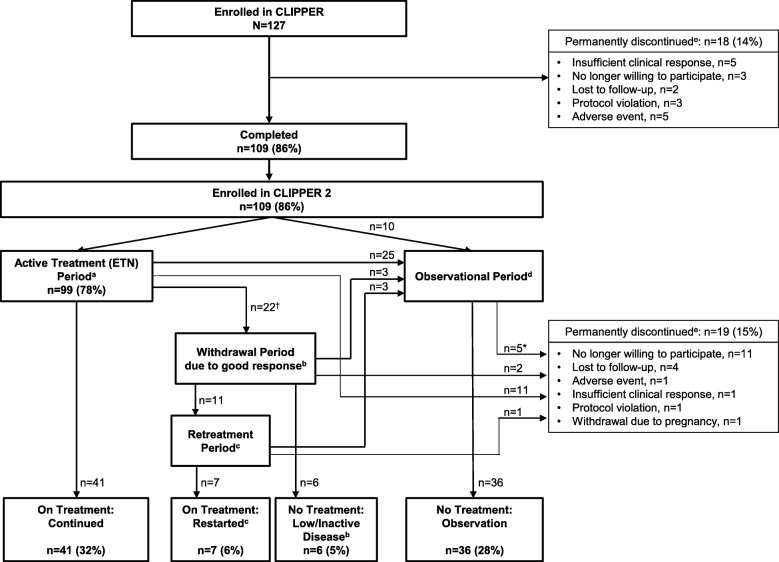
Fig. 1.
Study flow. (a) Patients actively receiving treatment with ETN. (b) Patients who either achieved CRACR or who, in the investigator’s clinical judgment, had a good clinical response and would benefit from treatment withdrawal. (c) Patients in the withdrawal period who required re-treatment per the investigator’s clinical judgment and re-started ETN. (d) Patients who stopped treatment but were still followed in CLIPPER2. (e) Patients who were no longer being followed as part of CLIPPER or CLIPPER2. *Includes two patients who entered the observational period directly from CLIPPER, plus three patients who entered the observational period from another treatment phase. †Nine out of 22 patients entered the withdrawal period because of a treatment-emergent adverse event. Abbreviations: CRACR, clinical remission, based on the American College of Rheumatology criteria [21]