Abstract
Background
Berberine is an isoquinoline alkaloid extracted from various Berberis species which is widely used in East Asia for a wide range of symptoms. Recently, neuroprotective effects of berberine in Alzheimer’s disease (AD) animal models are being extensively reported. So far, no clinical trial has been carried out on the neuroprotective effects of berberine. However, a review of the experimental data is needed before choosing berberine as a candidate drug for clinical experiments. We conducted a systematic review on AD rodent models to analyze the drug effects with minimal selection bias.
Methods
Five online literature databases were searched to find publications reporting studies of the effect of berberine treatment on animal models of AD. Up to March 2018, 15 papers were identified to describe the efficacy of berberine.
Results
The included 15 articles met our inclusion criteria with different quality ranging from 3 to 5. We analyzed data extracted from full texts with regard to pharmacological effects and potential anti-Alzheimer’s properties. Our analysis revealed that in multiple memory defects animal models, berberine showed significant memory-improving activities with multiple mechanisms, such as anti-inflammation, anti-oxidative stress, cholinesterase (ChE) inhibition and anti-amyloid effects.
Conclusion
AD is likely to be a complex disease driven by multiple factors. Yet, many therapeutic strategies based on lowering β-amyloid have failed in clinical trials. This suggest that the threapy should not base on a single cause of Alzheimer’s disease but rather a number of different pathways that lead to the disease. Overall we think that berberine can be a promising multipotent agent to combat Alzheimer’s disease.
Keywords: Berberine, Alzheimer’s disease, Neuroprotection, Dementia, Animal models
Background
Alzheimer’s disease (AD) is a progressive degenerative disease of the central nervous system. Its main clinical manifestations are progressive declines in memory and cognitive function, accompanied by psychiatric symptoms and abnormal behavior. AD mostly occurs in elderly persons over 65 years of age. According to 2017 statistics, there are nearly 46 million AD patients worldwide [1, 2]. In the brain, senile plaques (SP) and neurofibrillary tangles (NFT) are the diagnostic hall markers of AD. Its other pathological features include diffuse atrophy of the cortex, widening of the sulcus, enlargement of the ventricles, loss of neurons and decreases in choline acetylase and acetylcholine levels. The etiology of AD is still elusive, and several hypotheses have been proposed to explain the pathogenesis of AD. The most prevalent hypotheses are the amyloid β-protein (Aβ) cascade hypothesis [3, 4], hyper-phosphorylated Tau hypothesis [4], the free radical theory [5], the inflammatory theory [6] and cholinergic hypothesis [7]. The diversity and uncertainty of the pathogenesis of AD have caused difficulties in the development of effective treatment, and most of the clinical trials performed in recent decades have failed.
Berberine is an isoquinoline alkaloid that is widely present in several medicinal plants, especially in those belonging to the Berberis genus (e.g., Berberis vulgaris L., Berberidaceae). It also occurs, for example, in Coptis chinensis Franch. (Ranunculaceae), a plant which is used in traditional Chinese medicine as an anti-diarrheal, anti-bacterial, anti-fungal, and anti-protozoal agent, particularly in combination with other herbs [8–10]. The chemical structure of berberine is shown in Fig. 1. In several years, accumulating evidence has revealed a wide variety of bioactivities of berberine such as antiviral, antibacterial and anti-inflammatory [11, 12].
Fig. 1.
Chemical structures of berberine
The pharmacological effect of berberine on the nervous system was first reported in the 1970s as sedation-inducing [13]. The therapeutic activity of berberine has been widely examined in various neurological conditions including cerebral ischemic injury, AD, Parkinson’s disease, depression, anxiety, Huntington’s disease, epilepsy and convulsions. Several studies have shown that berberine can alleviate AD pathology through various mechanisms, including inhibition of hyper-phosphorylation of Tau protein and Aβ production. Berberine can reduce the hyper-phosphorylation of Tau protein, and this reduction may be related to the activation of the phosphatidylinositol 3-kinase/protein kinase/glycogen synthase kinase 3 pathway to restore protein phosphatase 2A activity and reverse glycogen synthase kinase-3 (GSK-3) activation [14]. In addition, berberine can inhibit the expression of beta-secretase by activating the extracellular signal-regulated kinase 1/2 signaling pathway, thereby inhibiting the production of Aβ40/42 [15]. Moreover, researchers have recently revealed that, on a molecular basis, berberine exerts inhibitory effects on the four key enzymes in the pathogenesis of AD: acetylcholinesterase, butyrylcholinesterase, monoamine oxidase A, and monoamine oxidase B [16].
Before this, several experiments have been performed to evaluate the anti-AD properties of berberine. However, these pre-clinical studies have not been systematically analyzed to provide a whole picture and un-biased understanding of the therapeutic potential of berberine for AD. The aim of this systematic review is to summarize the current evidence and analyze that evidence as to what it reveals about the underlying mechanism of the protective effects of berberine in animal AD models. We hope to provide more insightful information for future clinic trials.
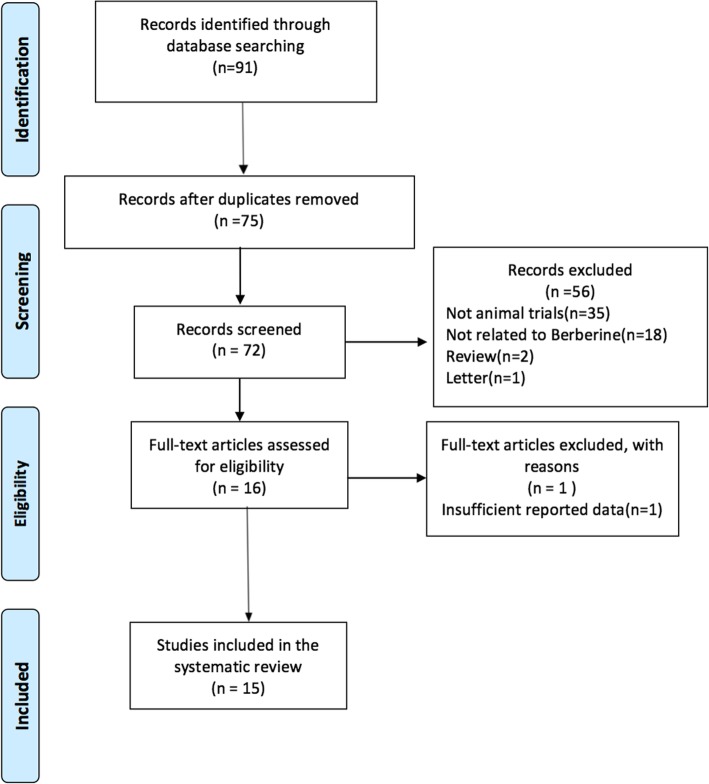
To perform the systematic review, we searched the literatures and selected the studies passing our selection criteria for data extraction and analysis. Our search of electronic databases returned a total of 91 articles. After deleting 16 which contained duplicated experimental data, we had a total of 72 references. After reading the titles and abstracts, we deleted 57 papers for the following reasons: (1) Not including experiments on animal models; (2) Not directly administering berberine; (3) No experimental details provided. Thus, finally, we had 15 articles that reported the efficacy of berberine in AD animal models; this review is based on these articles (Fig. 2).
Fig. 2.
Research methodology for review process
Method
Literature search
A careful literature search was performed to find publications reporting studies of the effect of berberine treatment on animal models of AD. Online literature databases (PubMed, Google scholar, PsychINFO, Embase and Web of Science) were searched up to March 2018 using search terms for English or Chinese publications. The following search strategy was used for each database.
Berberine
Alzheimer’s Disease
Alzheimer Disease
AD
or/2–4
1 and 5
Inclusion/exclusion criteria and screening
Inclusion criteria
Berberine was administered alone.
Experimental AD was induced in rodents (i.e., rats or mice).
AD treatment group was treated with a pharmacological agent, and a control group was administered a placebo after injury.
Article was published in English or Chinese.
Exclusion criteria
Not an original paper (review or letter etc.);
Berberine was not administered alone.
Absence of a correct control group.
Other types of animals (e.g., sheep, cats, and dogs) were used.
Duplicate publications.
Data extraction and quality assessment
Data extraction
Two investigators independently screened papers and listed them based on publication year, the first author’s name and experimental models. Using a structured form, they extracted individual data on study characteristics, methods and outcome measures. The differences in papers selected were resolved through discussion. Finally, the methodological quality of the included basic research was assessed by applying six correction scales.
Results
The search strategy retrieved 91 papers through online literature databases (PubMed, Google scholar, PsychINFO, Embase and Web of Science), 15 papers met our selection criteria. These 15 studies evaluated in this review involved animals from two species and four varieties: TgCRND8 mice, APP/PS1 mice, Sprague Dawley rats and Wister rats. The scales of the studies varied, from 6 to 104 animals in a single study. Rat and mouse weights were 200–300 g and 20–55 g, respectively. Eleven studies used male animals, and 1 study used female rats. After selecting and classifying these 15 studies, 3 were diabetic rat models with memory-impairment, 2 were 3 × Tg-AD mice models, 2 were Aβ infused rats models, 1 was an APP/PS1 mice model, 1 was a (Pilo)-induced epilepsy rat model, 1 was an ibotenic acid (ibo)-induced rat model and 5 memory-impairment models induced by Scopolamine, ICV-STZ, ethanol and D-galactose respectively. The research parameters evaluated in the 15 studies included the Morris water maze, immunohistochemistry (IHC), Western blot, RT-PCR (reverse transcription-polymerase chain reaction) and ELISA. The Morris water maze, a behavior test, was used to evaluate memory function. The IHC method as a molecular biology technique was used to investigate neuroprotective effects. Western blot, ELISA and RT-PCR techniques were used to measure potential genetic and proteins markers involved in Alzheimer’s disease. Table 1 lists the basic characteristics of the 15 studies.
Table 1.
Basic information of included studies
| Study | Animal Models | Administration(dosage, time and route) | Evaluation methods of the treatment effectiveness test |
|---|---|---|---|
| He et al. [17] | Thirty 120-day APP/PS1 mice (male, 30) | 0, 50 and 100 mg/kg-berberine (n = 10 for each group), intragastric administration for 14 days. | MWM, WB analysis, immunohistochemistry |
| Chen et al. [18] | Aged 4–5 weeks Wistar rats weighting about 200 g memory-impairment diabetic Wistar Rats model (male) | DM group (diabetes mellitus), berberine group (187.75 mg/kg/d) and Met group (Metformin, 184 mg/kg/d), orally administration for 2 weeks. | Fear Condition, PET, WB analysis, immunochemistry |
| Huang et al. [19] | 4-month 3 × Tg-AD (male,18;female,18) | 0, 50 and 100 mg/kg berberine (n = 12 for each group), orally administration for 4 months. | MWM, platform recognition, HVS water maze, Aβ1–42: Elisa, WB analysis, Immunofluorescence staining, histological analysis |
| Oliveira et al. [20] | 300–350 g ICV-STZ induce sporadic Alzheimer’s-like dementia Wistar rats model (male, 60) | Control (CTR), berberine 50 mg/kg, berberine 100 mg/kg, streptozotocin, streptozotocin plus berberine 50 mg/kg, and streptozotocin plus berberine 100 mg/kg, orally administration for 21 days. | MWM, Elevated plus maze task, LDH: Labtest kit, AChE activity |
| Patil et al. [21] | 200–250 g memory-impairment Wistar rats model induced by ethanol (male,12) | 25, 50 and 100 mg/kg berberine, vitamin C (100 mg/kg), and vehicle (1 mL/kg), DDW control rats(double distilled water), orally administration (chronic treatment: once a day for 45 days before training; acute treatment: once a day for 5 days during training). | MWM, Memory consolidation test, Elevated plus maze test, ChE activity |
| Haghani et al. [22] | 200–250 g 60–65 days Aβ infused Wistar rats model (male, 32) | Sham(normal saline), berberine(50 mg/kg), Aβ(normal saline)and Aβ + berberine(berberine 50 mg/kg) (n = 8 rats in each group), intraperitoneal administration daily for 13 days. | MWM, Passive avoidance test, |
| Zhan et al. [23] | 180–220 g, 10–12 weeks D-galactose-induced memory impairment Wistar rats (male) | Berberine (100 mg/kg per day), orally administration for 7 weeks. | MWM, WB analysis, RT-PCR |
| Gao et al. [24] | 200–250 g (Pilo)-induced epilepsy Sprague Dawley rats (male,104) | 1) control group (n = 12), saline; 2) berberine 100 mg/kg group (n = 12); 3) Pilo group (n = 20); 4) Pilo + berberine 25 mg/kg group (n = 20); 5) Pilo + berberine 50 mg/ kg group (n = 20); 6) Pilo + berberine 100 mg/ kg group (n = 20). Orally administration daily for 7 days before Pilo injection. | MWM, GSH level: sectrophotometrical |
| Moghaddam et al. [25] | (STZ)-diabetic Wistar rats (male,50) | Control, berberine-treated control (100 mg/kg;), diabetic, and berberine-treated diabetics (50 and 100 mg/kg respectively). | Y-maze, Single-trial passive avoidance test, Electrophysiological experiments |
| Durairajan et al. [15] | TgCRND8 mice | Berberine (25 mg/kg per day), berberine (100 mg/kg per day), orally administeration | MWM, ELISA, Immunohistochemistry, WB analyses |
| Lee et al. [26] | 260–280 g SCO-induced memory deficits SD rats (male) | SAL group, PA group, SCO-induced and saline-treated group, SCO + berberine20 mg/kg group,SCO + PA100 group, SCO-induced plus 200 mg/kg PA-treated group, and the SCO-induced plus 0.2 mg/kg TA-treated group. Intraperitoneally administration once a day for 14 days. | MWM, Hidden platform trial, Probe trial, immunohistochemical, ELISA, |
| Bhutada et al. [27] | Memory-impairment diabetic Wistar rats (male) |
1.berberine (25, 50, 100 mg/kg), vitamin C (100 mg/kg), metformin (500 mg/kg), or vehicle (1 mL/kg) twice daily for 30 days. Orally administration 2.berberine (25, 50, 100 mg/kg), vitamin C (100 mg/kg), metformin (500 mg/kg), vehicle (1 mL/kg), twice daily during training trials for next 5 days. Orally administration |
MWM, Memory consolidation test, Open field test |
| Lim et al. [28] | Ibotenic acid-induced memory deficient Sprague Dawley rat model (male) | IBO model(saline), IBO model(berberine), saline injected sham group(berberine), daily Intraperitoneally administration for a week. | immunohistochemistry |
| Zhu et al. [29] | injected A-beta (1–40) (5 microgram) AD rat model | Berberine chloride (50 mg/kg), Orally administration once daily for 14 days. | MWM, immunohistochemistry, PCR |
| Peng et al. [30] | 200–250 g SCOP-induced amnesia Sprague-Dawley rats (male) | Berberine (0.1 and 0.5 g/kg) orally daily for 7-day or 14-day. | Passive avoidance response task, Motor activity measurements |
MWM, Morris water maze; WB analysis, Western Blot analysis; PET, Positron-Emission Tomography; RT-PCR, Reverse Transcription Polymerase Chain Reaction; AchE, Acethyl-cholinesterase; ICV-STZ, Intracerebroventricular streptozotocin; GSH, glutathione; Pilo, pilocarpine;
Methodological quality
We assessed the scores of the quality according to these 6 points:
A: peer reviewed publication; B: random allocation to group; C: blinded assessment of outcome; D: a sample size calculation; E: compliance with animal welfare regulations; F: a statement of a potential conflict of interest.
The quality items scored in the included studies ranged from 3 to 5 out of a total of 6 points as shown in the Table 2. Two of the studies (13.3%) achieved 3 points; seven studies (46.7%) achieved 4 points; and Six studies (40%) achieved 5 points.
Table 2.
Methodological quality of included studies
| Study | A | B | C | D | E | F | Total |
|---|---|---|---|---|---|---|---|
| He et al. [20] | √ | √ | √ | √ | √ | 5 | |
| Chen et al. [27] | √ | √ | √ | √ | 4 | ||
| Huang et al. [21] | √ | √ | √ | √ | √ | 5 | |
| Oliveira et al. [19] | √ | √ | √ | √ | √ | 5 | |
| Patil et al. [17] | √ | √ | √ | √ | 4 | ||
| Haghani et al. [31] | √ | √ | √ | √ | √ | 5 | |
| Zhan et al. [32] | √ | √ | √ | √ | 4 | ||
| Gao et al. [22] | √ | √ | √ | √ | √ | 5 | |
| Moghaddam et al. [23] | √ | √ | √ | √ | 4 | ||
| Durairajan et al. [15] | √ | √ | √ | √ | √ | 5 | |
| Lee et al. [33] | √ | √ | √ | 3 | |||
| Bhutada et al. [34] | √ | √ | √ | 3 | |||
| Lim et al. [24] | √ | √ | √ | √ | 4 | ||
| Zhu et al. [25] | √ | √ | √ | √ | 4 | ||
| Peng et al. [28] | √ | √ | √ | √ | 4 |
Table 2 shows the Methodological quality of the 15 reviewed studies.
Anti-Alzheimer’s disease mechanisms of berberine
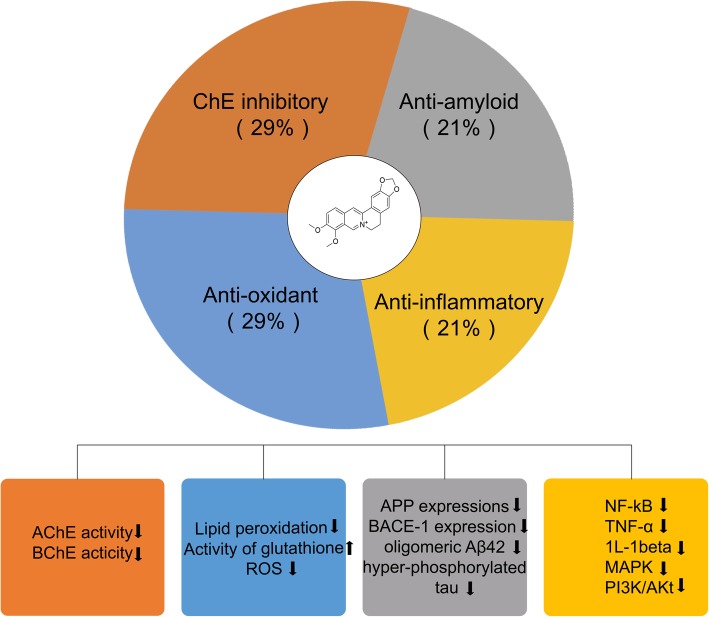
Table 3 shows the main outcomes and results of the included studies. Twelve studies investigated whether berberine improved cognitive abilities; four studies examined hippocampal cells of CA1 region and apoptosis of pyramidal neurons in the CA1 area. The changes in oxidative stress and acetylcholinesterase (AChE) activity were examined in 8 studies. Three studies tested NF-kB signaling. In addition, one study reported that berberine induced autophagy to reduce the APP and BACE1 levels. The above proposed neuroprotective mechanisms of berberine are summarized in Fig. 3.
Table 3.
Anti-AD effects and underlying mechanisms after berberine treatment of included studies
| Study | Anti-AD effects | Neuroprotection mechanism |
|---|---|---|
| He et al. [17] |
1. Mitigated cognitive impairment of AD mice. 2. Inhibited phosphorylation of Tau. 3. Limited lipid peroxidation. 4. Lowered the levels of IL-1b and TNF-a. 5. Lowered the levels of both GFAP and CD45. |
Anti-inflammatory, suppressed NF-kB signaling pathway, anti-oxidative stress and anti-apoptosis. |
| Chen et al. [18] |
1. Inhibited the inflammation mediator release and insulin resistance in the mPFC of diabetic rats.2. Ameliorated cognitive impairment and accelerates the reinforcement of the information. 3. Decreased the expressions of amyloid precursor protein and BACE-1, and the production of oligomeric Aβ42. |
Inhibits the PI3K/Akt/mTOR and MAPK signaling pathway, as well as two novel isoforms PKCη and PKCε and the translocation of NF-κB Anti-inflammatory. Anti-amyloid. |
| Huang et al. [19] | 1. Ameliorated cognitive deficits, improved spatial learning capacity and memory retention in 3 × Tg-AD mice model2. Reduced the production of Aβ and BACE1 protein level in primary hippocampal neurons and the brains of 3 × Tg-AD mice | Anti-amyloid, enhancing autophagy through the class III PI3K/beclin-1 pathway. Anti-apoptosis. |
| Oliveira et al. [20] |
1. Prevented the memory loss, anxiogenic behavior 2. Reduced escape latency in ICV-STZ rats 3. Reduced the number of dead cells in both the hippocampus and cerebral cortex in STZ rat. 4. Decreased AChE activity in both the hippocampus and cerebral cortex of ICV-STZ rat. |
ChE inhibition. |
| Patil et al. [21] |
1. Improved ethanol-induced memory impairment. 2. Lowered oxidative stress and ChE activity in ethanol treated rats. |
Anti-oxidant and ChE inhibition. |
| Haghani et al. [22] |
1. Prevented the impairing impacts of Aβ on the learning, memory and electrophysiological properties of the CA1 pyramidal neurons. 2. Improved the memory performance. 3. Restored the Aβ-induced impairments in the firing frequency, half-width and rebound action potential. |
Phenomenon research. |
| Zhan et al. [23] | 1. Rescued D-galactose-induced memory impairment the mRNA and protein levels of Arc/Arg3.12. Reversed the synaptic deficits induced by D-galactose. | Phenomenon research. |
| Gao et al. [24] |
1. Relieved pilocarpine-induced convulsions in rats. 2. Reduced the degree of oxidative stress in the hippocampus.3. Attenuated memory impairment. 4. alleviated neuronal degeneration in hippocampal CA1 region in SE rats. |
Anti-oxidant. |
| Moghaddam et al. [25] | 1. Ameliorated learning and memory impairment.2. Restored PS amplitude and fEPSP and ameliorated learning and memory impairment and attenuated apoptosis of pyramidal neurons in the CA1 area. | Anti-apoptosis. |
| Durairajan et al. [15] |
1. Lower Aβ levels, alleviated cognitive deficits and amyloid neuropathology, reduce gliosis. 2. Reduced the Aβ and CTFs, probably by downregulating the phosphorylation of APP and of CTFs via the activation of the PI3K/Akt/GSK3 pathway. 3. Reduced the cognitive impairment 4. Decreased Aβ plaques of all 3 size subsets (25, 25–50, and > 50 μm). 5. Reduced vascular amyloids as well as parenchymal amyloids 6. Reducing ThioS-positive vascular amyloids. 7. 45% reduction in microgliosis and a 54% decrease in astrocytosis |
Anti-amyloid, activation of the PI3K/Akt/GSK3 pathway |
| Lee et al. [26] | 1. Improved memory impairment and reduced the escape latency.2. Alleviated memory-associated decreases and restored brain-derived neurotrophic factor and cAMP-response element-binding protein mRNA expression in the hippocampus.3. Decreases the expression of proinflammatory cytokines mRNA in the hippocampus. | Anti-inflammatory. |
| Bhutada et al. [27] |
1. Improved cognitive performance. 2. Lowered hyperglycemia, oxidative stress, and ChE activity in diabetic rats. |
Anti-oxidant. ChE inhibition. |
| Lim et al. [28] |
1. Increased neuronal cells immunoreactive to calbindin in the hippocampus and entorhinal cortex area. 2. Hippocampal cells were increased in the pyramidal layer of CA1 region and dentate gyrus |
Phenomenon research. |
| Zhu et al. [29] | Ameliorates the spatial memory impairment. | Phenomenon research. |
| Peng et al. [30] | Improved SCO-induced amnesia | Phenomenon research. |
ChE, cholinesterase; IL-1β, Interleukin 1 beta; TNF-α, Tumor necrosis factor alpha; GFAP, Glial fibrillary acidic protein; CD45, CD45 Antigen; mPFC, The medial prefrontal cortex; BACE-1, β-site amyloid precursor protein cleaving enzyme 1; ICV-STZ, Intracerebroventricular streptozotocin; NF-kB, Nuclear factor kappa-light-chain-enhancer of activated B cells; MAPK, Mitogen-activated protein kinases; PKCη, Protein kinase C-eta type; PKCε, Protein kinase C epsilon type; fEPSP, Field excitatory postsynaptic potential; cAMP, Cyclic adenosine monophosphate
Fig. 3.
Reported potential mechanisms underlying anti-AD property of berberine
Discussion
Potential mechanisms underlying anti-Alzheimer’s disease properties of berberine
The neuroprotective effects of berberine have been extensively studied in different animal experimental models and we summarized the studies which include a rat model of amyloid beta induced-Alzheimer’s disease, a memory impairment model induced by ethanol in rats, a D-galactose-induced memory deficits model in rats, a pilocarpine (Pilo)-induced epilepsy model in rats, a scopolamine and streptozotocin-induced memory impairment model in rats, a memory-deficient rat model induced by stereotaxic injection of ibotenic acid into entorhinal cortex (Ibo model), and the transgenic mouse model of Alzheimer’s disease. Interestingly, berberine displayed significant effects in preventing memory impairment in these mechanistically different animal models, suggesting an over-all improvement of memory function by berberine. Indeed, mechanistic studies showed that berberine modulated a wide range of biological functions to exert neuroprotection and the detailed mechanisms are discussed in the following part.
Antioxidant properties of berberine
Alzheimer’s disease is characterized by extensive evidence of oxidative stress which is the result of uncontrolled production of reactive oxygen species (ROS) [35]. ROS has been regarded as a critical factor in the neuron dysfunction or death of neuronal cells that contribute to the pathogenesis of the disease [36]. Under normal conditions, the damage caused by oxygen free radicals can be controlled through a series of reactive antioxidant systems. However, under pathological conditions, the balance between oxidants and antioxidants is disturbed such that active oxygen production exceeds cellular antioxidant defenses. The antioxidant activity of berberine has been widely demonstrated [34, 37–39]. For instance, berberine displayed peroxynitrite (ONOO−) scavenging activity and total ROS inhibitory capacities [37]. Bhutada et al. [27] showed that berberine treatment during training trials also improved learning and memory, lowered hyperglycemia, oxidative stress, and ChE activity in diabetic rats.
-
(b)
Anti-inflammatory properties of berberine
In the brain of patients with Alzheimer’s disease, chronic inflammation has been well described. On the histological level, this inflammation is characterized by activated microglia, reactive astrocytes and increased inflammatory cytokines release [33]. This observation has led to the hypothesis that brain inflammation is a cause of neuronal damage in AD and anti-inflammatory drugs may be used as protective agents. Chen et al. [18] studied the functions of berberine involved in anti-inflammation and the amelioration of insulin resistance in the prefrontal cortex of diabetic rats. They found that intragastric administration of berberine (187.5 mg/kg/d) inhibited inflammation mediator release and insulin resistance in the mPFC of diabetic rats. Finally, it relieved the impairment of cognitive function in diabetic rats. The promising effect of Phellodendron amurense (PA) and its major alkaloid compound, berberine, on memory dysfunction has also been studied in scopolamine-induced memory deficient rats [26]. A two-week administration of 20 mg/ kg of berberine improved memory impairment as measured by the passive avoidance test, and it reduced the escape latency for finding the platform in the Morris water maze test.
-
(c)
Anti-cholinesterase activity of berberine
The cholinergic hypothesis was initially presented several years ago, then several studies demonstrated the adverse effects of anticholinergic drugs on memory [40], the low intracerebral cholinergic activity in patients with Alzheimer’s disease (AD) [41, 42] and the association of AD with cholinergic transmission disorders [43]. This hypothesis suggests that decreased cholinergic activity is associated with the AD symptoms and the improvement of cholinergic activity will relieve the AD symptoms. The cholinesterase (ChE) is the major enzyme for acetylcholine destruction and its inhibition results in increasing acetylcholine level in the brain. Therefore, many anti-AD pharmacological studies have focused on cholinesterase (ChE) inhibitors to ameliorate the cognitive symptoms [44]. Several studies have been performed to examine the effect of berberine on the ChE activity. For example, chronic treatment with berberine (25–100 mg/kg) lowered oxidative stress and ChE activity in ethanol treated rats [21]. A similar promising effect of one-month treatment with berberine on streptozotocin-induced memory impairment in rats has been reported [20]. In another set of experiments, berberine (100 mg/kg) treatment during training trials also improved learning and memory and lowered hyperglycemia, oxidative stress, and ChE activity [27].
-
(d)
Anti-amyloid activity of berberine
The 42-amino acid amyloid beta (Aβ) is released from cleavage of the amyloid precursor protein by β-secretase and γ-secretase [45]. The Aβ sequenced from the meningeal blood vessels of AD patients and individuals with Downs’ syndrome is highly aggregated, and spontaneously assumes the β-sheet conformation and polymerizes into oligomers, fibrils, fibrils and plaques [46]. Berberine has been shown to ameliorates β-amyloid pathology and cognitive impairment in an AD transgenic mouse model [19]. After berberine treatment, the levels of extracellular and intracellular Aβ1–42 were decreased, mediated by increased autophagy activity.
With advances in science, there is increasing interest in another constituent of neurofibrillary tangles(NFTs), hyper-phosphorylated Tau protein. He et al. found that berberine improved learning and memory in APP/PS1 mice, decreased hyper-phosphorylated Tau protein and lowered the activity of NF-kB signaling in the hippocampus of APP/PS1 mice [17]. Berberine administration promoted the activity of glutathione (GSH) and inhibited lipid peroxidation in the hippocampus of AD mice. They concluded that berberine attenuated cognitive deficits and limited hyper-phosphorylation of Tau via inhibiting the activation of the NF-kB signaling pathway and by retarding oxidative stress and neuro-inflammation.
Opportunities and challenges
Berberine is a natural product with a definite structure and a wide range of pharmacological effects. Berberine displays many biological functions and potential therapeutic applications in neurological diseases. Animal research is an essential early step toward evaluating and developing an intervention for clinical trials in humans [31]. This systematic review has examined high quality animal studies on the anti-AD effects of berberine and finds a consistent effect of berberine in improving the memory defects in multiple animal models, indicating the therapeutic potential of berberine for treating AD. While the effects are clear, the mechanism is not; further research is needed to determine the details of the biochemical mechanisms and specific drug target(s). Meanwhile, perhaps the greatest barrier to the pharmaceutical development of berberine is its naturally low bioavailability. More effort, for example, in structural modification and/or pharmaceutical processing, is needed for berberine to achieve its full potential in clinical use [32]. The following suggestions are worth considering: 1. The feasibility of targeted drug delivery should be explored. It is difficult to achieve effective concentrations, especially in the brain, by oral administration so targeted administration is worth considering; 2. The effects of berberine in combination with other drugs for AD treatment can be tested. 3. The possibility of toxic effects of berberine during long-term drug administration must be considered, and thoroughly studied.
Conclusions
In this paper, we have reviewed 15 high-quality animal studies on the neuroprotective effects of berberine against AD, with systematic evaluation of its efficacy and pharmacology mechanisms. Berberine has showed significant memory-improving activities in multiple memory defects animal models; common properties, including anti-oxidation, anti-inflammation and anti-ChE activity were revealed. So far, no clinical trial has been carried out on the neuroprotective effects of berberine. Considering the positive results from animal studies and the relatively low toxicity of berberine, the performance of clinical trials to evaluate the anti-AD effect of berberine on human patients appears justified.
Acknowledgements
Not applicable.
Funding
This study was supported by the Grants of China NSFC-31500831, Macau government grants FDCT-022/2015/A1, FDCT-024-2017-AMJ, and SKL-QRCM-2014-2016, and the University of Macau grant MYRG2016-0019-ICMS-QRCM, and the joint grant EF001/ICMS-LJH/2016/HKBU awarded to Jiahong Lu.
Availability of data and materials
The datasets and materials supporting the conclusions of this article are presented in this main paper.
Abbreviations
- AchE
Acethyl-cholinesterase
- AD
Alzheimer’s disease
- BACE-1
β-site amyloid precursor protein cleaving enzyme 1
- BChE
Butyrylcholinesterase
- cAMP
Cyclic adenosine monophosphate
- CD45
CD45 Antigen
- fEPSP
Field excitatory postsynaptic potential
- GFAP
Glial fibrillary acidic protein
- ICV-STZ
Intracerebroventricular streptozotocin
- IL-1β
Interleukin 1 beta
- MAPK
Mitogen-activated protein kinases
- mPFC
The medial prefrontal cortex
- MWM
Morris water maze
- NF-kB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PET
Positron-Emission Tomography
- PKCε
Protein kinase C epsilon type
- PKCη
Protein kinase C-eta type
- RT-PCR
Reverse Transcription Polymerase Chain Reaction
- TNF-α
Tumor necrosis factor alpha
- WB analysis
Western Blot analysis
Authors’ contributions
All listed authors worked collectively on the design of the study and development of the review instruments. YNN and CCZ carried out article reviews, data extraction, analysis of the data, and drafted the manuscript. WMY carried out article reviews and worked on the paper search methodology. SHX oversaw the database searches, and carried out all the article tabulation. ML and LJH participated in development of the review instruments and advised on statistical conduct of the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ning-Ning Yuan, Email: ningning.yuan@yahoo.com.
Cui-Zan Cai, Email: caizan617@126.com.
Ming-Yue Wu, Email: blackmoon19890103@hotmail.com.
Huan-Xing Su, Email: huanxingsu@umac.mo.
Min Li, Email: limin@hkbu.edu.hk.
Jia-Hong Lu, Phone: 853-8822-4508, Email: jiahonglu@umcac.mo.
References
- 1.Krantic S. Editorial (thematic issue: from current diagnostic tools and therapeutics for Alzheimer's disease towards earlier diagnostic markers and treatment targets) Curr Alzheimer Res. 2017;14:2–5. doi: 10.2174/156720501401161201104858. [DOI] [PubMed] [Google Scholar]
- 2.Association As 2017 Alzheimer's disease facts and figures. Alzheimers Dement. 2017;13:325–373. doi: 10.1016/j.jalz.2017.02.001. [DOI] [Google Scholar]
- 3.Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-V. [DOI] [PubMed] [Google Scholar]
- 4.Mudher A, Lovestone S. Alzheimer's disease–do tauists and baptists finally shake hands? Trends Neurosci. 2002;25:22–26. doi: 10.1016/S0166-2236(00)02031-2. [DOI] [PubMed] [Google Scholar]
- 5.Su B, Wang X, Nunomura A, Moreira PI, Lee H-g, Perry G, Smith MA, Zhu X. Oxidative stress signaling in Alzheimer's disease. Curr Alzheimer Res. 2008;5:525–532. doi: 10.2174/156720508786898451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greig NH, Mattson MP, Perry T, Chan SL, Giordano T, Sambamurti K, Rogers JT, Ovadia H, Lahiri DK. New therapeutic strategies and drug candidates for neurodegenerative diseases: p53 and TNF-α inhibitors, and GLP-1 receptor agonists. Ann N Y Acad Sci. 2004;1035:290–315. doi: 10.1196/annals.1332.018. [DOI] [PubMed] [Google Scholar]
- 7.Terry AV, Buccafusco J. The cholinergic hypothesis of age and Alzheimer's disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther. 2003;306:821–827. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- 8.Mirska I, Kedzia H, Kowalewski Z, Kedzia W. The effect of berberine sulfate on healthy mice infected with Candida albicans. Arch Immunol Ther Exp. 1972;20:921–929. [PubMed] [Google Scholar]
- 9.Yamamoto K, Takase H, Abe K, Saito Y, Suzuki A. Pharmacological studies on antidiarrheal effects of a preparation containing berberine and geranii herba. Nihon yakurigaku zasshi Folia pharmacologica Japonica. 1993;101:169–175. doi: 10.1254/fpj.101.3_169. [DOI] [PubMed] [Google Scholar]
- 10.Ou M. Chinese–English manual of common used in traditional Chinese medicine. Hong Kong: Guangdong Science and Technology Publishing House. In: Joint Publishing (HK); 1999.
- 11.Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res. 2008;22:999–1012. doi: 10.1002/ptr.2399. [DOI] [PubMed] [Google Scholar]
- 12.Kuo C-L, Chi C-W, Liu T-Y. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004;203:127–137. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Shanbhag S, KULKARNI HJ, Gaitonde B. Pharmacological actions of berberine on the central nervous system. Jpn J Pharmacol. 1970;20:482–487. doi: 10.1254/jjp.20.482. [DOI] [PubMed] [Google Scholar]
- 14.Yu G, Li Y, Tian Q, Liu R, Wang Q, Wang J-Z, Wang X. Berberine attenuates calyculin A-induced cytotoxicity and tau hyperphosphorylation in HEK293 cells. J Alzheimers Dis. 2011;24:525–535. doi: 10.3233/JAD-2011-101779. [DOI] [PubMed] [Google Scholar]
- 15.Durairajan SSK, Liu L-F, Lu J-H, Chen L-L, Yuan Q, Chung SK, Huang L, Li X-S, Huang J-D, Li M. Berberine ameliorates β-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer's disease transgenic mouse model. Neurobiol Aging. 2012;33:2903–2919. doi: 10.1016/j.neurobiolaging.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Ji H-F, Shen L. Molecular basis of inhibitory activities of berberine against pathogenic enzymes in Alzheimer's disease. Sci World J. 2012;2012. [DOI] [PMC free article] [PubMed]
- 17.He W, Wang C, Chen Y, He Y, Cai Z. Berberine attenuates cognitive impairment and ameliorates tau hyperphosphorylation by limiting the self-perpetuating pathogenic cycle between NF-κB signaling, oxidative stress and neuroinflammation. Pharmacol Rep. 2017;69:1341–1348. doi: 10.1016/j.pharep.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Chen Q, Mo R, Wu N, Zou X, Shi C, Gong J, Li J, Fang K, Wang D, Yang D. Berberine ameliorates diabetes-associated cognitive decline through modulation of aberrant inflammation response and insulin signaling pathway in DM rats. Front Pharmacol. 2017;8:334. doi: 10.3389/fphar.2017.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang M, Jiang X, Liang Y, Liu Q, Chen S, Guo Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of β-amyloid in APP/tau/PS1 mouse model of Alzheimer's disease. Exp Gerontol. 2017;91:25–33. doi: 10.1016/j.exger.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 20.de Oliveira JS, Abdalla FH, Dornelles GL, Adefegha SA, Palma TV, Signor C, da Silva BJ, Baldissarelli J, Lenz LS, Magni LP. Berberine protects against memory impairment and anxiogenic-like behavior in rats submitted to sporadic Alzheimer’s-like dementia: involvement of acetylcholinesterase and cell death. Neurotoxicology. 2016;57:241–250. doi: 10.1016/j.neuro.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Patil S, Tawari S, Mundhada D, Nadeem S. Protective effect of berberine, an isoquinoline alkaloid ameliorates ethanol-induced oxidative stress and memory dysfunction in rats. Pharmacol Biochem Behav. 2015;136:13–20. doi: 10.1016/j.pbb.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Haghani M, Shabani M, Tondar M. The therapeutic potential of berberine against the altered intrinsic properties of the CA1 neurons induced by Aβ neurotoxicity. Eur J Pharmacol. 2015;758:82–88. doi: 10.1016/j.ejphar.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Zhan P-Y, Peng C-X, Zhang L-H. Berberine rescues D-galactose-induced synaptic/memory impairment by regulating the levels of arc. Pharmacol Biochem Behav. 2014;117:47–51. doi: 10.1016/j.pbb.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Gao F, Gao Y, Liu Y-F, Wang L, Li Y-J. Berberine exerts an anticonvulsant effect and ameliorates memory impairment and oxidative stress in a pilocarpine-induced epilepsy model in the rat. Neuropsychiatr Dis Treat. 2014;10:2139. doi: 10.2147/NDT.S73210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalalian-Moghaddam H, Baluchnejadmojarad T, Roghani M, Goshadrou F, Ronaghi A. Hippocampal synaptic plasticity restoration and anti-apoptotic effect underlie berberine improvement of learning and memory in streptozotocin-diabetic rats. Eur J Pharmacol. 2013;698:259–266. doi: 10.1016/j.ejphar.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Lee B, Sur B, Shim I, Lee H, Hahm D-H. Phellodendron amurense and its major alkaloid compound, berberine ameliorates scopolamine-induced neuronal impairment and memory dysfunction in rats. Korean J Physiol Pha. 2012;16:79–89. doi: 10.4196/kjpp.2012.16.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhutada P, Mundhada Y, Bansod K, Tawari S, Patil S, Dixit P, Umathe S, Mundhada D. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav Brain Res. 2011;220:30–41. doi: 10.1016/j.bbr.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Lim JS, Kim H, Choi Y, Kwon H, Shin KS, Joung I, Shin M, Kwon YK. Neuroprotective effects of berberine in neurodegeneration model rats induced by ibotenic acid. Anim Cells Syst. 2008;12:203–209. doi: 10.1080/19768354.2008.9647174. [DOI] [Google Scholar]
- 29.Zhu F, Qian C. Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin-1beta and inducible nitric oxide synthase in the rat model of Alzheimer's disease. BMC Neurosci. 2006;7:78. doi: 10.1186/1471-2202-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng W-H, Hsieh M-T, Wu C-R. Effect of long-term administration of berberine on scopolamine-induced amnesia in rats. Jpn J Pharmacol. 1997;74:261–266. doi: 10.1254/jjp.74.261. [DOI] [PubMed] [Google Scholar]
- 31.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silverman RB, Holladay MW. The organic chemistry of drug design and drug action: academic press. 2014. [Google Scholar]
- 33.Rogers J, Webster S, Lue L-F, Brachova L, Civin WH, Emmerling M, Shivers B, Walker D, McGeer P. Inflammation and Alzheimer's disease pathogenesis. Neurobiol Aging. 1996;17:681–686. doi: 10.1016/0197-4580(96)00115-7. [DOI] [PubMed] [Google Scholar]
- 34.Sarna LK, Wu N, Hwang S-Y, Siow YL, O K. Berberine inhibits NADPH oxidase mediated superoxide anion production in macrophages. Can J Physiol Pharmacol 2010; 88: 369–378. [DOI] [PubMed]
- 35.Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- 36.Markesbery WR. The role of oxidative stress in Alzheimer disease. ARCH Neurol-Chicago. 1999;56:1449–1452. doi: 10.1001/archneur.56.12.1449. [DOI] [PubMed] [Google Scholar]
- 37.Jung HA, Min B-S, Yokozawa T, Lee J-H, Kim YS, Choi JS. Anti-Alzheimer and antioxidant activities of Coptidis Rhizoma alkaloids. Biol Pharm Bull. 2009;32:1433–1438. doi: 10.1248/bpb.32.1433. [DOI] [PubMed] [Google Scholar]
- 38.Yokozawa T, Ishida A, Cho EJ, Kim HY, Kashiwada Y, Ikeshiro Y. Coptidis Rhizoma: protective effects against peroxynitrite-induced oxidative damage and elucidation of its active components. J Pharm Pharmacol. 2004;56:547–556. doi: 10.1211/0022357023024. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh Y-S, Kuo W-H, Lin T-W, Chang H-R, Lin T-H, Chen P-N, Chu S-C. Protective effects of berberine against low-density lipoprotein (LDL) oxidation and oxidized LDL-induced cytotoxicity on endothelial cells. J Agric Food Chem. 2007;55:10437–10445. doi: 10.1021/jf071868c. [DOI] [PubMed] [Google Scholar]
- 40.Drachman DA, Leavitt J. Human memory and the cholinergic system: a relationship to aging? Arch Neurol-Chicago. 1974;30:113–121. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- 41.Bowen D, Allen S, Benton J, Goodhardt M, Haan E, Palmer A, Sims N, Smith C, Spillane J, Esiri M. Biochemical assessment of serotonergic and cholinergic dysfunction and cerebral atrophy in Alzheimer's disease. JNeur. 1983;41:266–272. doi: 10.1111/j.1471-4159.1983.tb11838.x. [DOI] [PubMed] [Google Scholar]
- 42.Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Sci. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 43.Coyle JT, Price DL, Delong MR. Alzheimer's disease: a disorder of cortical cholinergic innervation. Sci. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 44.Cummings JL. Cholinesterase inhibitors: a new class of psychotropic compounds. Am J Psychiatry. 2000;157:4–15. doi: 10.1176/ajp.157.1.4. [DOI] [PubMed] [Google Scholar]
- 45.Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. BBRC. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 46.Cai X-D, Golde TE, Younkin SG. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Sci. 1993;259:514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets and materials supporting the conclusions of this article are presented in this main paper.