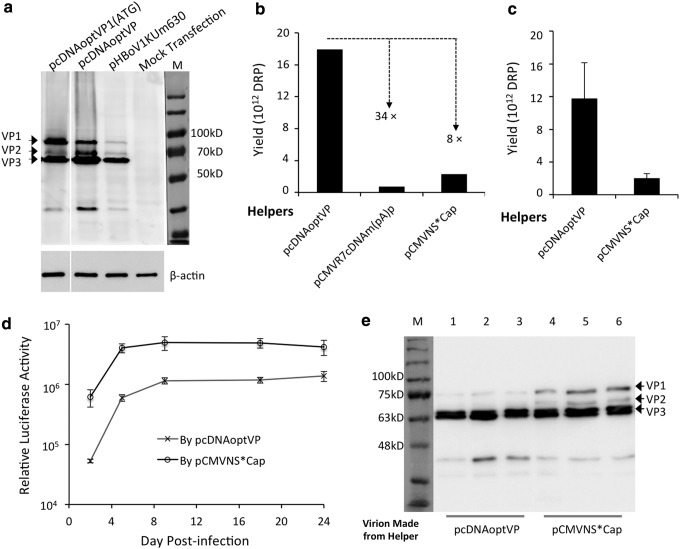
Figure 3.
Effectiveness of NS-free rAAV2/HBoV1 production system. (a) Comparison of the expression of HBoV1 capsid proteins in transfected HEK293 cells. Expression of VP1, VP2, and VP3 was assessed by Western blot analysis following transfection with codon-optimized HBoV1 cap in helper pcDNAoptVP1(ATG), pcDNAoptVP, or replication-incompetent HBoV1 genome clone pHBoV1KUm630. Cell lysates from the transfections were resolved in same polyacrylamide gel, but several irrelevant lanes were removed. After probing for HBoV1 capsid proteins, the blot was re-probed for β-actin expression. (b and c) Quantitation of vector production. (b) Representative example of a side-by-side comparison of production yields from indicated HBoV1 helper plasmids for forty 150 mm plates of transfected HEK293 cells. (c) Comparison of average yields for NS-free helper pcDNAoptVP and NP1-depedent helper pCMVNS*Cap. Values represent mean ± SEM yield from four side-by-side preparations. (d) Comparison of transduction activities of rAAV2/HBoV1 preparations produced using pcDNAoptVP and pCMVNS*Cap. HAE-ALI cultures apically infected with AV2/HBc-gLuc at an MOI of 10,000 DRP/cell. Values represent mean ± SEM (n = 4) accumulated gaussia luciferase activity for the previous 24 h period at each time point. (e) Comparison of the content of HBoV1 capsid subunits in virions, as assessed by Western blot analysis. 1.3 × 1010 DRP of virions were loaded per lane. Lanes 1–3: virions generated using helper pcDNAoptVP. Lanes 4–6: virions generated using helper pCMVNS*Cap. VP1, VP2, and VP3 bands were detected using anti-HBoV1 VP3 antibody and visualized by horseradish peroxidase–conjugated anti-rat immunoglobulin G antibody. Note: Anti-HBoV1 VP3 visualized a low-molecular weight band on the blots from the transfection of capsid expression plasmid (a) and also from purified virions (e). It is unclear if this band is a degradative product or an unknown small VP component incorporated in the virions.