Figure 2. Uncontrolled activation of myostatin/activing causes muscle wasting in patients with cancer.
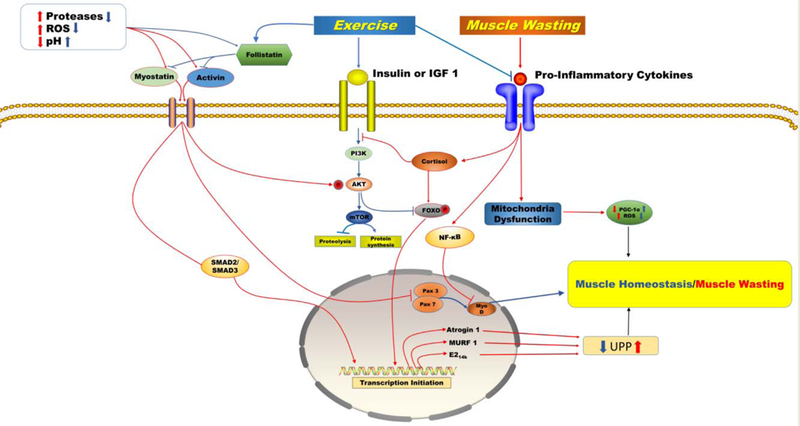
An increase in extracellular proteases and free radicals accompanied by a severe decrease in pH and follistatin activates myostatin/activin factors. In myofibers, binding of myostatin/activin to their receptors activates downstream processes that inhibit the IGF-1/PI3K/Akt hypertrophy pathway through phosphorylation of Akt, resulting in the translocation of smad 2/3 and FoxO1 into the nucleus and the upregulation of transcription of proteolytic genes, and inhibits myoblast growth and myogenic differentiation through suppression of Pax3/7 and myoD. This cycle of events initiates muscle wasting by upregulating transcription of Atrogin 1, MURF 1, and E2 ligases, which are all active in the ubiquitin proteasome pathway. A chronic inflammatory response leads to altered Hypothalamus Pituitary Adrenal axis signalling causing a dysregulated cortisol response that induces muscle wasting through a resistance to insulin and IGF-1, activation and translocation of NF-κB into the nucleus, and mitochondria dysfunction. Exercise improves muscle wasting by mediating key pathways. Exercise regulates myostatin/activin by reducing proteases and free radicals while increasing pH and follistatin. Exercise activates the PI3K/Akt/mTOR pathway. This pathway inhibits FOXO through phosphorylation. Exercise reduces local and systemic inflammation, regulates cortisol production, and enhances mitochondria biogenesis and myocellular regeneration while reducing production of reactive oxygen species which results in muscle homeostasis. Blue arrows describe the pathways to muscle homeostasis under conditions of chronic exercise. Red arrows describe the pathways to muscle wasting in patients with advanced staged cancer
