Abstract
Background
The contribution of immune activation to arterial stiffness and its reversibility in human immunodeficiency virus (HIV)–infected adults in sub-Saharan Africa is unknown.
Methods
HIV-uninfected and HIV-infected Malawian adults initiating antiretroviral therapy (ART) with a CD4+ T-cell count of <100 cells/μL were enrolled and followed for 44 weeks; enrollment of infected adults occurred 2 weeks after ART initiation. We evaluated the relationship between carotid femoral pulse wave velocity (cfPWV) and T-cell activation (defined as HLA-DR+CD38+ T cells), exhaustion (define as PD-1+ T cells), and senescence (defined as CD57+ T cells) and monocyte subsets, using normal regression.
Results
In 279 HIV-infected and 110 HIV-uninfected adults, 142 (37%) had hypertension. HIV was independently associated with a 12% higher cfPWV (P = .02) at baseline and a 14% higher cfPWV at week 10 (P = .02), but the increases resolved by week 22. CD4+ and CD8+ T-cell exhaustion were independently associated with a higher cfPWV at baseline (P = .02). At 44 weeks, arterial stiffness improved more in those with greater decreases in the percentage of CD8+ T cells and the percentage of PD-1+CD8+ T cells (P = .01 and P = .03, respectively). When considering HIV-infected participants alone, the adjusted arterial stiffness at week 44 tended to be lower in those with higher baseline percentage of PD-1+CD8+ T cells (P = .054).
Conclusions
PD-1+CD8+ T-cells are associated with HIV-related arterial stiffness, which remains elevated during the first 3 months of ART. Resources to prevent cardiovascular disease in sub-Saharan Africa should focus on blood pressure reduction and individuals with a low CD4+ T-cell count during early ART.
Keywords: HIV, PD-1, T-cell exhaustion, sub-Saharan Africa, endothelial damage, arterial stiffness, cardiovascular disease
Arterial stiffness is increased in Malawian adults with low CD4 during the first 3 months of antiretroviral therapy, compared to adults without HIV. Hypertension is an important traditional risk factor and immune activation, including CD8 exhaustion, also contributes to arterial stiffness.
Sub-Saharan Africa (SSA) has the highest burden of human immunodeficiency virus (HIV) infection worldwide, with 25.6 million people living with the disease [1]. The region also faces an accelerated epidemic of noncommunicable diseases, including cardiovascular disease (CVD) [2]. Mortality from CVD is high in SSA, causing an estimated 1 million deaths/year [3]. This rate is predicted to increase, with noncommunicable disease–related mortality estimated to surpass infection-related deaths by 2030 [3]. Studies from high-income settings suggest the risk of CVD is approximately doubled in people living with HIV, even after adjustment for confounders such as socioeconomic status, traditional cardiovascular risk factors, and viral hepatitis virus coinfection [4, 5]. Several processes may contribute, including a high HIV load; side effects of antiretroviral therapy (ART), particularly protease inhibitor treatment; and the effects of chronic immune activation despite effective ART [6]. Immune activation may be driven by persistent low-level HIV viremia, microbial translocation, and subclinical infections [7, 8]. However, the risk of CVD in people living with HIV in SSA has not been well characterized. It is likely that immune activation differs in low-income settings, owing to the effects of more-advanced HIV disease at presentation, more-frequent acute coinfections, and malnutrition with disruption to the gut barrier [9, 10]. Traditional cardiovascular risk factors also vary, with hypertension being more prevalent than diabetes, dyslipidemia, or obesity, although with epidemiological transition and increasing urbanization in the region, this may change in the near future [11, 12].
Large cohorts documenting cardiovascular events have not been established in SSA, limiting the assessment of cardiovascular risk. One physiological marker of cardiovascular risk is the carotid femoral pulse wave velocity (cfPWV), a gold standard measurement of arterial stiffness [13–15]. Although adjusted for concurrent blood pressure, the reading can be affected by blood viscosity and ambient temperature [13]. Nevertheless it has been shown to be reliable and reproducible and has been validated against clinical outcomes in high-income settings [16–18]; a cfPWV in the top versus bottom tertile is associated with a >2-fold increased risk of myocardial infarction/stroke [18]. The 2007 European Society of Cardiology consensus guidelines proposed a 12-m/second threshold as high risk for CVD events [14]. A few small studies have assessed arterial stiffness in people living with HIV in low-income areas of SSA [19–23], but none have evaluated the impact of chronic immune activation over time. This study therefore aimed to characterize the contribution of immune activation to arterial stiffness in HIV-infected Malawian adults initiating ART with advanced immunosuppression, compared with that in HIV-uninfected adults, and to determine how this changed over time on ART.
METHODS
Study Design
Adults aged >18 years presenting for HIV testing at the voluntary testing clinic, the HIV outpatient clinic, and the medical inpatient wards at the Queen Elizabeth Central Hospital (Blantyre, Malawi) were recruited into a prospective cohort from January 2014 until June 2015. Adults with a new HIV diagnosis were approached consecutively and were eligible if they were ART naive, had a CD4+ T-cell count of <100 cells/μL, and provided informed written consent. Adults who were confirmed to be uninfected with HIV after self-presenting for an asymptomatic HIV test at the same voluntary testing clinic were eligible if they had no current illness; no history of infection in the previous month, based on clinician assessment and medical notes review; and provided informed written consent. Exclusion criteria were living outside the Blantyre area, inability to attend follow-up visits, pregnancy, or being too unwell to participate, as judged by the study clinicians. Because HIV-uninfected participants were younger, an exclusion criterion of <35 years of age was used for this group from March 2014 onward. From January 2014 until January 2015, HIV-infected participants were corecruited with the REALITY trial (clinical trials registration NCT01825031). REALITY assessed interventions to reduce early mortality following ART initiation in those with a CD4+ T-cell count of <100 cells/µL. Participants were simultaneously randomly assigned to one of 3 study interventions, in addition to standard triple-drug ART and cotrimoxazole, and also received a 12-week supply adjunctive raltegravir, a package of opportunistic infection prophylaxis, and/or ready-to-use supplementary food [24–26].
At enrollment and 44 weeks later, participants underwent a detailed clinical assessment, including evaluation for traditional cardiovascular risk factors and infection history and collection of fasting blood specimens, with some specimens collected in tubes containing sodium citrate for immunophenotyping. To reduce the burden of study participation and to ensure that starting ART was prioritized in the severely immunosuppressed HIV-infected population, the baseline study visit was conducted at week 2 following ART initiation. cfPWV was assessed for all participants at enrollment and 10, 22, and 44 weeks later. All participants provided informed written consent, and ethical approval was granted by the College of Medicine Research and Ethics Committee, University of Malawi (P.09/13/1464), and by the University of Liverpool Research and Ethics Committee (UoL000996).
Outcome Measurement
cfPWV was measured using a Vicorder device (Skidmore Medical, London, UK). The distance was the length from the sternal notch to the umbilicus and then the top mid-point of the femoral cuff, multiplied by 0.8 as per consensus guidelines. Wave forms were saved, and a random sample was reviewed by an experienced independent assessor, blinded to HIV status, at 3 time points during the study, to ensure consistent quality. The intraoperator concordance correlation coefficient for 10 participants was 0.99 (95% confidence interval [CI], .96–1.00).
Immunophenotyping of Peripheral Blood Mononuclear Cells (PBMCs)
For flow cytometry, whole-blood specimens were processed within 4 hours of collection to isolate PBMCs, using Lymphoprep (Axis-Shields-Diagnostics) as previously described [27]. Cells were analyzed using a CyAn ADP 9 color flow cytometer (Beckman Coulter). The T-cell panel included CD3 BV510, CD4 V450, CD38 PE Cy7, HLA-DR AF700, PD-1 APC, and CD57 FITC (all from BD Biosciences) and CD8 PE (Biolegend). The monocyte panel included HLA-DR AF700, CD14 PE Cy7, and CD16 PE (all from BD Biosciences). Anti-mouse Igk isotype control and negative control particles (BD Biosciences) were used for compensation. A standardized gating strategy was followed for T cells (Supplementary Figure 1A) and monocytes (Supplementary Figure 1B). Monocyte subsets were identified as previously described [28].
Statistical Analysis
As data validating a clinically relevant cfPWV threshold in SSA were not available, the 12-m/second threshold in European consensus guidance was used to guide sample size calculations [14]. Recruiting 300 HIV-infected and 100 HIV-uninfected participants provided 80% power to detect an odds ratio (OR) of 1.5 associated with HIV, assuming that 25% of HIV-uninfected participants had cfPWV >12 m/second [14]. After enrollment, it was clear that this threshold was rarely reached, and therefore the planned analysis considered the primary outcome, cfPWV, as a continuous variable.
Factors affecting HIV and arterial stiffness were identified a priori, using a causal diagram (Supplementary Figure 2), as either potential mediators (ie, on the mechanistic pathway between them) or confounders (ie, associated with HIV and arterial stiffness but not on the mechanistic pathway). Categorical and continuous variables were compared between HIV-infected and uninfected adults by using χ2 and rank-sum tests, respectively. Correlations between continuous variables were compared using Spearman rho. To avoid undue influence from outliers, continuous variables were truncated at their 97.5th and 2.5th percentiles for regression models. Normal linear regression models were constructed, with log10 cfPWV (approximately normal) as the outcome and HIV infection as the primary exposure. Confounders and mediators with a univariate P value of <.2 for association with cfPWV were considered, and backward elimination (exit P = .2) was used to identify a final model. Where 2 variables were strongly collinear, the variable with the strongest univariate association with cfPWV was considered for inclusion in the multivariate model. Independent effects of immunophenotyping parameters on this model were then also assessed. Overall changes in log10 cfPWV over the first 44 weeks from enrollment were estimated using random effects models, considering the impact of HIV on cfPWV both at baseline (intercept) and over time (interaction with time). Regression models for cfPWV 44 weeks after enrollment in HIV-infected participants adjusted for baseline cfPWV and factors identified as confounders or mediators at baseline, and they additionally considered the impact of immune parameters. Adjustment for baseline means that these models identify predictors of change from baseline.
All analysis was undertaken using Stata v13.1 (Statacorp, College Station, TX).
RESULTS
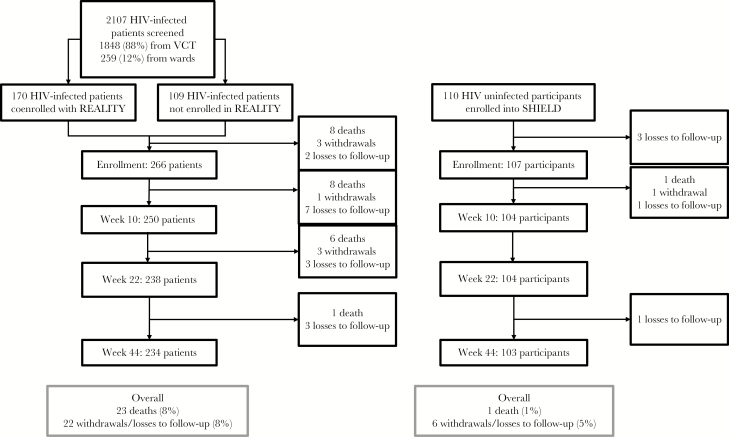
A total of 2107 adults with a new HIV diagnosis were screened, of whom 279 (13%) were recruited. One hundred seventy (61%) were corecruited with the REALITY trial, and 109 (39%) were not (Figure 1). Most exclusions (involving 1477 adults [70%]) were because of a CD4+ T-cell count of >100 cells/mm3 (complete list of exclusions is in Supplementary Table 1). One hundred ten HIV-uninfected adults were also recruited. Although the HIV-infected and uninfected groups were of similar age (median, 36.6 versus 34.8 years; Table 1), HIV-infected participants were more likely to be male and to have a lower level of education. Three HIV-uninfected adults (3%) versus 5 HIV-infected adults (2%) had a diagnosis of hypertension at enrollment; a further 46 (42%) and 88 (32%), respectively, were discovered to have hypertension during the study. HIV-infected participants had advanced immunosuppression (median CD4+ T-cell count, 41 cells/μL; median HIV load, 5.06 log10 copies/mL), although only 54 (19%) had World Health Organization (WHO) HIV disease stage 3 or 4.
Figure 1.
Recruitment flow. Enrollment was performed 2 weeks after screening. HIV, human immunodeficiency virus; VCT, voluntary counseling and testing.
Table 1.
Baseline Characteristics, According to Human Immunodeficiency Virus (HIV) Status
| Characteristic | Complete Cases | HIV Uninfected (n = 110) | HIV Infected (n = 279) | P |
|---|---|---|---|---|
| Demographic variable | ||||
| Age | 388 | 34.8 (30.8–41.2) | 36.6 (31.1–43.3) | .41 |
| Female sex | 389 | 66 (60) | 122 (44) | .004 |
| Primary school education or less | 352 | 38 (40) | 136 (53) | .024 |
| Traditional cardiovascular risk factor | ||||
| Weight, kg | 375 | 60 (53–68) | 53 (48–59) | <.0001 |
| Waist to height ratioa | 349 | 0.47 (0.44–0.53) | 0.45 (0.43–0.49) | .0003 |
| BMIb | 373 | 21.7 (20.2–25.2) | 19.8 (18.3–21.9) | <.0001 |
| Blood pressure, mm Hg | ||||
| Systolic | 361 | 128 (114–134) | 120 (108–128) | .0001 |
| Diastolic | 358 | 75 (68–82) | 73 (68–80) | .27 |
| History of smokingc | 389 | 16 (15) | 56 (20) | .21 |
| History of alcohol usec | 389 | 28 (25) | 119 (43) | .002 |
| Preexisting CVD diagnosis | 370 | 1 (1) | 1 (0.4) | .47 |
| Prescribed CVD medications | 370 | 5 (5) | 4 (1.5) | .08 |
| Preexisting diabetes | 367 | 1 (1.0) | 1 (0.4) | .65 |
| Preexisting hypertension | 366 | 3 (3.0) | 5 (2.0) | .40 |
| New diagnosis of hypertension | 358 | 46 (42) | 88 (32) | .055 |
| Fasting cholesterol level, mmol/L | 377 | 4.0 (3.3–4.5) | 3.6 (3.0–4.4) | .049 |
| Fasting glucose level, mmol/L | 327 | 4.6 (4.2–5.2) | 4.9 (4.4–5.6) | .015 |
| Creatinine level, µmol/L | 381 | 62 (54–71) | 65 (54–78) | .13 |
| Infection-related factor | ||||
| Heart rate, beats/min | 360 | 72 (68–80) | 82 (72–98) | <.0001 |
| Hemoglobin level, g/dL | 375 | 13.8 (12.7–14.7) | 11.4 (10.0–13.0) | <.0001 |
| Current infectious disease at enrollment | 377 | 3 (3)d | 57 (21) | <.0001 |
| Tuberculosis | … | 0 (0) | 2 (1) | |
| Cryptococcal meningitis | … | 0 (0) | 0 (0) | |
| Pneumonia | … | 0 (0) | 10 (4) | |
| Gastroenteritis | … | 1 (1) | 17 (6) | |
| Malaria | … | 2 (2) | 3 (1) | |
| Immune-related factor | ||||
| Lymphocyte count, ×109 cells/L | 370 | 2.1 (1.6–2.6) | 1.2 (0.8–1.7) | <.0001 |
| Monocyte count, ×109 cells/L | 323 | 0.30 (0.25–0.50) | 0.40 (0.22–0.60) | .053 |
| Absolute CD4+ T-cell count, cells/µL | … | … | 41 (18–62) | |
| HIV load, log10 copies/mL | … | … | 5.06 (4.62–5.47) | |
| T-cell activation,e % of cells | ||||
| CD4+ T cells | 193 | 5 (3–9) | 22 (11–34) | <.0001 |
| CD8+ T cells | 290 | 11 (6–19) | 34 (21–49) | <.0001 |
| T-cell senescence,f % of cells | ||||
| CD4+ T cells | 194 | 7 (4–9) | 15 (9–24) | .0001 |
| CD8+ T cells | 295 | 40 (27–53) | 54 (44–64) | <.0001 |
| Monocyte phenotype | ||||
| Classical (CD14++CD16−) | 263 | 75 (65–81) | 76 (66–83) | .79 |
| Intermediate (CD14++CD16+) | 263 | 9 (7–14) | 10 (6–13) | .10 |
| Nonclassical (CD14+CD16+) | 263 | 13 (10–22) | 14 (9–21) | .59 |
Data are median value (interquartile range) or no. (%) of participants.
Abbreviations: BP, blood pressure; CVD, cardiovascular disease.
aWaist to height ratio measures central obesity, a risk factor for metabolic syndrome [47].
bBody mass index (BMI) is calculated as the ratio of the weight in kilograms divided by the height in square meters.
cDefined as either a past or current history of regular alcohol or smoking.
dBased on results of tests returned after enrollment and at which point the participants reported and physicians confirmed the absence of infection.
eDefined as CD38+HLA-DR+ cells.
fDefined as PD-1+ cells.
All but 1 HIV-infected participant initiated standard first-line therapy with tenofovir-lamivudine-efavirenz (the exception received zidovudine-lamivudine-nevirapine). In total, 28 (7%) participants withdrew or were lost to follow-up, and 24 (6%) died. Thirteen (29%) were lost to follow-up or died within the first 2 weeks; 16 (36%), between 2 and 10 weeks; and 16 (36%), after 10 weeks (Figure 1). One HIV-uninfected participant died from a hypertension-related intracranial bleed. The 23 deaths in HIV-infected participants were due to pulmonary/disseminated tuberculosis (6), cryptococcal meningitis (3), Kaposi sarcoma (3), gastroenteritis (1), and tuberculous meningitis (1). The cause of death was unknown for 9 participants.
Arterial Stiffness at Enrollment
At enrollment, the median cfPWV was 7.3 m/second (interquartile range [IQR], 6.5–8.2 m/second) in HIV-infected participants versus 7.2 m/second (IQR, 6.2–8.0 m/second) in uninfected participants (P = .07). cfPWV was >12 m/second for 5 patients, of whom 4 had HIV infection.
Immune Activation at Enrollment
As expected, compared with HIV-uninfected participants, HIV-infected adults had higher proportions of activated (CD38+HLA-DR+) CD4+ and CD8+ T cells (P < .0001 for both comparisons). HIV-infected adults also had a higher proportion of exhausted (PD-1+) CD4+ and CD8+ T cells (P < .0001 for both comparisons). In contrast, there were no differences between HIV-infected and uninfected participants in classical (CD14++CD16−), intermediate (CD14++CD16+), and nonclassical (CD14+CD16+) monocytes (P = .79, .10, and .59, respectively; Table 1).
T-Cell PD-1 Expression Is Independently Associated With HIV-Related Arterial Stiffness 2 Weeks After ART Initiation
Factors univariately associated with baseline cfPWV with a P value of < .2 are shown in Table 2A; neither WHO stage nor a diagnosis of acute coinfection were associated with baseline cfPWV (P = .25 and .23, respectively). Independently, every 10-year increase in age was associated with an 18% increase in cfPWV (95% CI, 14%–23%; P < .0001; univariable associations are in Supplementary Figure 3), with no evidence of an effect modification between age and HIV (interaction P = .73). Every 10–mm Hg increase in diastolic blood pressure was also associated with a 9% increase in cfPWV (95% CI, 4%–13%; P < .0001), and women had a 9% lower cfPWV (95% CI, 2%–16%) than men (P = .001).
Table 2A.
Predictors of Carotid Femoral Pulse Wave Velocity (cfPWV) Among Adult Malawians at Enrollment
| Predictor | Evaluable Adults, No. | Univariate Analysis | Multivariate Analysis Including Confounders (n = 353 Complete Cases) |
Multivariate Analysis Including Mediators and Confounders (n = 335 Complete Cases) |
|||
|---|---|---|---|---|---|---|---|
| cfPWV,b Fold Change (95% CI) | P | cfPWV,b Fold Change (95% CI) | P | cfPWV,b Fold Change (95% CI) | P | ||
| HIV infection | 366 | 1.09 (.44–2.68) | .06 | 1.07 (.99–1.16) | .08 | 1.12 (1.02–1.23) | .02 |
| Potential confounderb | |||||||
| Age (per 10-y increase) | 366 | 1.23 (1.18–1.27) | <.0001 | 1.18 (1.14–1.23) | <.0001 | 1.18 (1.13–1.23) | <.0001 |
| Female sex (vs male sex) | 366 | 0.87 (.80–.94) | .001 | 0.91 (.84–.98) | .01 | 0.94 (.86–1.02) | .15 |
| Diastolic BP (per 10–mm Hg increase) | 353 | 1.13 (1.09–1.18) | <.0001 | 1.09 (1.04–1.13) | <.0001 | 1.07 (1.03–1.13) | .001 |
| Potential mediatorb | |||||||
| Hemoglobin level (per 1-g/dL increase) | 355 | 1.02 (1.00–1.03) | .07 | … | 1.02 (1.00–1.04) | .07 | |
| Weight (per 10-kg increase) | 363 | 1.05 (1.01–1.09) | .02 | … | 1.01 (.97–1.05) | .58 | |
| Cholesterol level (per 1-mmol/L increase) | 357 | 1.04 (1.00–1.08) | .09 | … | 0.99 (.95–1.03) | .62 | |
| Recent acute coinfection | 364 | 1.11 (.99–1.25) | .08 | … | 1.09 (.98–1.22) | .11 |
aThe log10 cfPWV was the outcome in linear regression models, providing model coefficients that correspond to fold (relative) changes when back transformed. This table reflects the inclusion of all relevant variables with a P value of < .2 for the association with cfPWV in univariable analyses. There was no association between cfPWV and CD4+ T-cell activation (P = .77), CD8+ T-cell activation (P = .37), CD4+ T-cell senescence (P = .98), and CD8+ T-cell senescence (P = .15).
bSee Supplementary Figure 2 for the directed acyclic graph and identification of confounders vs mediators.
Abbreviations: BP, blood pressure; CI, confidence interval; HIV, human immunodeficiency virus.
After adjustment for these confounders (Table 2A), there was weak evidence that HIV-infected participants had a 7% greater cfPWV (95% CI, −1%–16%; P = .08). After adjustment for confounders and mediators, HIV was significantly associated with cfPWV, with a 12% greater cfPWV (95% CI, 2%–23%) in HIV-infected participants (P = .02; Table 2A). The effect of sex weakened with the addition of potential mediators (ie, hemoglobin level, weight, cholesterol level, and recent infection) to the model, but effects of age and diastolic blood pressure remained. cfPWV increased by 2% with every 1-g/dL increase in hemoglobin level (P = .07), which may be a marker of plasma viscosity. Concurrent infection at HIV diagnosis was not associated with cfPWV (adjusted fold change, 9%; 95% CI, −2%–21%; P = .13). When immune variables were considered in addition to this model (Table 2B), exhausted CD4+ and CD8+ T cells were each independently associated with cfPWV (P = .02), and the independent effect of HIV was lost. HIV remained significantly associated with cfPWV, excluding those with WHO stage 3 and 4 (fold change, 12%; 95% CI, 3%–24%; P = .01).
Table 2B.
Effect of the Addition of T-Cell Exhaustion Markers on the Relationship Between Human Immunodeficiency Virus (HIV) and Carotid Femoral Pulse Wave Velocity (cfPWV)
| Predictor | Traditional Risk Factors Model | |||||
|---|---|---|---|---|---|---|
| With HIV Status (n = 335) | With CD4+ T-Cell Exhaustion (n = 181) | With CD8+ T-Cell Exhaustion (n = 270) | ||||
| cfPWV,a Fold Change (95% CI) | P | cfPWV,a Fold Change (95% CI) | P | cfPWV,a Fold Change (95% CI) | P | |
| Age (per 10-y increase) | 1.18 (1.13–1.23) | <.0001 | 1.15 (1.10–1.21) | <.0001 | 1.15 (1.10–1.20) | <.0001 |
| Female sex (vs male sex) | 0.92 (.85–.99) | .02 | 0.83 (.74–.92) | <.0001 | 0.88 (.81–.96) | .006 |
| Diastolic BP (per 10–mm Hg increase) | 1.07 (1.03–1.13) | .001 | 1.10 (1.04–1.17) | .01 | 1.10 (1.05–1.15) | <.0001 |
| Hemoglobin level (per 1-g/dL increase) | 1.02 (1.00–1.04) | .07 | 1.01 (.99–1.04) | .36 | 1.01 (.99–1.03) | .21 |
| HIV infection | 1.12 (1.02–1.23) | .02 | 1.00 (.86–1.15) | .96 | 1.05 (.94–1.17) | .40 |
| CD4+ T-cell exhaustion % (per 10-pp increase) | … | 1.03 (1.00–1.05) | .02 | … | ||
| CD8+ T-cell exhaustion % (per 10-pp increase) | … | … | 1.03 (1.00–1.05) | .02 |
Abbreviations: BP, blood pressure; CI, confidence interval; HIV, human immunodeficiency virus; pp, percentage point.
aThe log10 cfPWV was the outcome in linear regression models, providing model coefficients that correspond to fold (relative) changes when back transformed. This table reflects the use of backward elimination to select a final model from confounders and mediators in Table 2A and then considers additional effects of CD4+ or CD8+ T-cell exhaustion (there were similar effects in a model including both; n = 178). There was no association between cfPWV and CD4+ T-cell activation (P = .77), CD8+ T-cell activation (P = .37), CD4+ T-cell senescence (P = .98), and CD8+ T-cell senescence (P = .15).
HIV-Related Arterial Stiffness Improves During ART
At week 44, 228 participants with (82%) and 103 (94%) without HIV infection remained in the study. All HIV-uninfected participants were retested for HIV at the week 44 visit, and none had acquired a new infection.
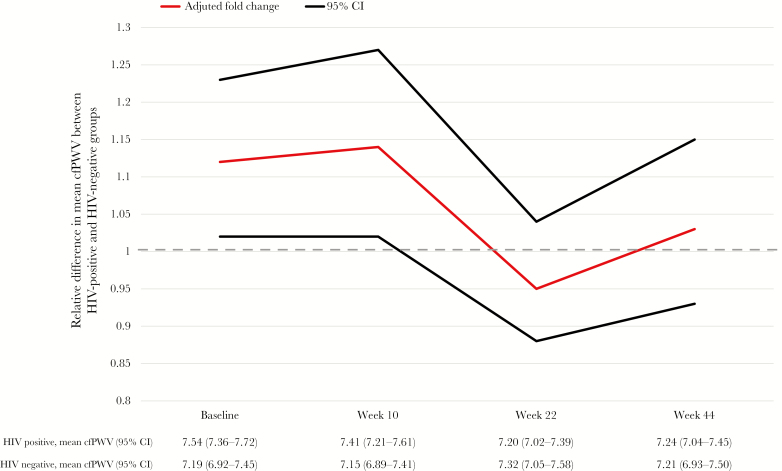
HIV-infected participants still had a significantly higher cfPWV at week 10 (adjusted P = .02) but not at week 22 (P = .46) or week 44 (P = .59; Figure 2). Overall, from enrollment through 44 weeks, cfPWV declined by 9% (95% CI, 4%–15%; P = .002) in HIV-infected participants but did not change significantly in HIV-uninfected participants (change, 2%; 95% CI, −7%–12%; P = .69; heterogeneity P = .04). In a sensitivity analysis, a similar effect of HIV on cfPWV was found at each time point when only including participants who completed the study: fold change at enrollment, 10% (95% CI, 0%–21%); fold change at week 10, 13% (95% CI, 2%–27%; P = .022); fold change at week 24, −4% (95% CI, −13%–6%; P = .45); and fold change at week 44, 3% (95% CI, −7%–14%; P = .60).
Figure 2.
Effect of human immunodeficiency virus (HIV) infection on the carotid femoral pulse wave velocity (cfPWV) over 44 weeks after adjustment for age, sex, diastolic blood pressure, and hemoglobin level. The same model is calculated for each individual time point. CI, confidence interval.
For HIV-infected participants, the median CD4+ T-cell count increased to 144 cells/μL (IQR, 99–218 cells/μL) at week 44. The mean percentage of CD8+ T cells decreased significantly (from 82% [95% CI, 84%–96%] to 60% [95% CI, 54%–67%]; P < .0001). The only significant decreases by week 44 involved the mean percentage of activated CD4+ T cells (from 74% [95% CI, 62%–86%] to 61% [95% CI, 50%–69%]; P < .0001) and the mean percentages of exhausted CD4+ T cells (from 54% [95% CI, 31%–67%) to 32% [95% CI, 22%–48%]; P < .0001) and exhausted CD8+ T cells (from 38% [95% CI, 29%–50%] to 32% [95% CI, 21%–47%]; P = .007; Supplementary Table 2).
Resolution of Higher CD8+ T-Cell PD-1 Expression Is Associated With Lower Arterial Stiffness 44 Weeks After ART Initiation
Next, we examined the impact of baseline factors on cfPWV 44 weeks after ART initiation, adjusting for baseline cfPWV (equivalent to predictors for the change in cfPWV), age, sex, baseline hemoglobin level, and diastolic blood pressure (Table 3). There was a trend toward an independent association between a higher percentage of PD-1+CD8+ T cells at baseline and a lower arterial stiffness 44 weeks after ART initiation (4% decrease in the cfPWV at week 44 for every 10% increase in the percentage of PD-1+CD8+ T cells at enrollment; P = .054). When REALITY intervention arms were added one by one to the same model (adjusted for age, sex, baseline hemoglobin level, diastolic blood pressure, PD-1+CD8+ T-cell percentage, and baseline cfPWV), there was no evidence of an association between the week 44 cfPWV and random assignment to enhanced infection prophylaxis (fold change vs standard-prophylaxis, 2%; 95% CI, −9%–11%; P = .76) or to enhanced nutritional support (fold change vs standard support, 8%; 95% CI, −3%–20%; P = .15). However, there was a trend toward a lower adjusted cfPWV at week 44 among the 82 participants (29%) randomly assigned to adjunctive raltegravir for 12 weeks at ART initiation (fold change vs standard ART alone, −11%; 95% CI, −21% to −1%; P = .04). This effect of raltegravir on the cfPWV at week 44 was not lost by adding the HIV load at baseline (fold change, −12%; 95% CI, −22%–0%; P = .05), week 12 (fold change, −14%; 95% CI, −25%–0%; P = .04), or week 24 (fold change, −12%; 95% CI, −24%–1%; P = .08).
Table 3.
Factors Associated With Arterial Stiffness 44 Weeks After Antiretroviral Therapy Initiation Among Human Immunodeficiency Virus–Infected Adults
| Factor | Baseline Factors Only (n = 237) | Baseline Factors and Change From Baseline to Week 44a (n = 174) | ||
|---|---|---|---|---|
| Fold Change (95% CI) | P | Fold Change (95% CI) | P | |
| Baseline cfPWV (per 1-m/second increase) | 2.70 (1.83–4.00) | <.0001 | 1.15 (1.09–1.22) | <.0001 |
| Age (per 10-y increase) | 1.20 (1.11–1.30) | <.0001 | 1.21 (1.11–1.32) | <.0001 |
| Baseline hemoglobin level (per 1-g/dL increase) | 1.03 (1.00–1.06) | .037 | 1.04 (1.00–1.07) | .027 |
| Baseline PD-1+CD8+ T-cell % (per 10-pp increase) | 0.96 (.91–1.00) | .054 | 0.99 (.95–1.03) | .71 |
| Change in PD-1+CD8+ T-cell % over 44 wk (per 10-pp increase) | … | 1.02 (1.00–1.05) | .079 | |
| Change in CD8+ T-cell % over 44 wk (per 10-pp increase) | … | 1.04 (.99–1.09) | .13 |
Abbreviations: CI, confidence interval; cfPWV, carotid femoral pulse wave velocity; pp, percentage point.
aIncluding factors with a univariable P value of < .05 from Table 4.
Examining the relationship between the change in immune markers and the change in the cfPWV from baseline to week 44, we found that greater decreases in the percentage of CD8+ T cells, the percentage of PD-1+CD8+ T cells, and the proportion of intermediate monocytes were associated with greater improvements in arterial stiffness univariably (P = .01, P = .03, and P = .054, respectively; Table 4). Adjusting for baseline factors and changes in the percentage of CD8+ T cells and the percentage of PD-1+CD8+ T cells, we found that the baseline effect of the percentage of PD-1+CD8+ T cells on the cfPWV at week 44 was attenuated and that there was instead a trend toward a lower cfPWV at week 44 among participants with a greater decrease in the percentage of PD-1+CD8+ T cells (P = .079; Table 3). Overall, these data suggest that resolution of an initially high proportion of PD-1+CD8+ T cells is associated with an improvement in arterial stiffness over 44 weeks of ART.
Table 4.
Change in Clinical and Immune Parameters and Association With Change in Arterial Stiffness 44 Weeks After Enrollment Among Human Immunodeficiency Virus (HIV)–Infected Adults
| Parameter | Rho | P |
|---|---|---|
| Blood pressure (mm Hg) | ||
| Systolic | 0.03 | .72 |
| Diastolic | 0.07 | .29 |
| Weight (kg) | −0.09 | .21 |
| Creatinine level (µmol/L) | 0.01 | .88 |
| Hemoglobin level (g/dL) | 0.03 | .72 |
| HIV load (copies/µL) | −0.08 | .28 |
| CD4+ T-cell count (cells/µL) | −0.04 | .64 |
| CD8+ T-cell % | 0.21 | .01 |
| CD4+ to CD8+ T-cell ratio | −0.13 | .12 |
| CD4+ T-cell parameter (% of cells) | ||
| Activated | 0.03 | .79 |
| Exhausted | 0.14 | .22 |
| Senescent | 0.13 | .24 |
| CD8+ T-cell parameter (% of cells) | ||
| Activated | −0.03 | .72 |
| Exhausted | 0.19 | .03 |
| Senescent | 0.04 | .67 |
| Monocyte phenotype (% of cells) | ||
| Classical | −0.16 | .07 |
| Intermediate | 0.18 | .054 |
| Nonclassical | 0.01 | .91 |
DISCUSSION
We have demonstrated that arterial stiffness is increased in Malawian adults with advanced HIV disease during the first 3 months of ART and that T cells expressing PD-1 are associated with this effect. Further, we have shown that this effect is reversible, in a cohort initiating ART with severe immunosuppression and with only modest increases in CD4+ T-cell counts during ART. Those with the highest proportion of PD-1+CD8+ T cells seemed to benefit the most from ART, demonstrating lower arterial stiffness 44 weeks after ART initiation. This is superimposed on a high background prevalence of hypertension.
Hypertension was the most important traditional risk factor for cardiovascular disease [29]. Our findings are consistent with results of studies from the region, which reported a prevalence of 30%–50% in the general population, with as few as 7% aware of their diagnosis [30]. In regions further along the epidemiological transition [11], lifestyle-related CVD risk factors such as obesity and diabetes are becoming more important and are compounded by HIV infection [31]. Intervention to prevent CVD in low-income countries is urgently needed before traditional risk factors intersect with the increased risk associated with HIV.
HIV-infected participants had a 12% higher adjusted arterial stiffness at ART initiation, compared with our HIV-uninfected population. Persistent higher arterial stiffness in the first 3 months of ART is consistent with findings by Benjamin et al, who reported vasculitis as the pathological phenotype of vascular injury during this period, potentially reflecting an immune reconstitution inflammatory syndrome (IRIS)–type phenomenon [32]. Excess mortality during the first 3 months of ART is well recognized in patients initiating ART with very low CD4+ T-cell counts [10, 33]. The REALITY trial assessed interventions to reduce this, but even with enhanced infection prophylaxis, 24-week mortality was still 8.9% [34]. Vascular inflammation, driven by high immune activation, may contribute to some of the adverse events seen during the first 3 months of ART in those initiating ART with low CD4+ T-cell counts.
In particular, PD-1+CD8+ T cells have previously been associated with endothelial dysfunction in patients with HIV infection [35–38]. In a cross-sectional study of 358 participants from the SCOPE cohort (of whom 75% had achieved virological suppression during ART), PD-1+ T cells were raised during untreated and treated HIV infection, and PD-1+CD8+ expression was particularly associated with markers of HIV antigenemia, including CD8+ T-cell activation and the HIV load [39]. Given the association with reductions in arterial stiffness demonstrated here, the PD-1+CD8+ expression pathway warrants further investigation. However, in an environment where concurrent acute and latent infections are common, it may be that improvements in PD-1+CD8+ expression and arterial stiffness were not due to ART and control of HIV alone [40, 41]. Rather, there may have been a protective effect from coadministration of trimethoprim-sulfamethoxazole, preventing coinfections such as those due to malaria parasite or bacteria. A so-called ART care effect may have contributed to improvements over the study period, whereby patients who are engaged in care in a low-income setting experience benefits such as frequent monitoring or better access to care.
Study strengths include prospective follow-up with robust assessment of clinical and cardiovascular measures, as well as a comprehensive longitudinal characterization of both monocyte and T-cell surface activation markers from fresh PBMCs in a large cohort of HIV-infected and uninfected participants. As HIV infection is a generalized epidemic in Malawi, the HIV-infected population is likely to have a traditional cardiovascular risk profile broadly similar to that of HIV-uninfected adults enrolled from the same facility. Further, all but 1 participant received the same standard first-line ART regimen, meaning that choices relating to the specific ART regimen cannot be confounders of the associations identified.
Limitations include the fact that cfPWV has not been validated to predict cardiovascular events in low-income areas of SSA; however, cfPWV has been validated robustly elsewhere [42, 43]. The relatively small differences identified in continuous cfPWV may have uncertain clinical relevance, and our hypothesized poor outcome (cfPWV >12 m/second) was rare, likely because our power calculations were based on studies from high-income settings and on much older populations than the HIV-infected population in SSA [44–46]. Overall, our study is still the largest to address this issue in the region, and it demonstrates that HIV has an effect on the risk of cardiovascular events that is similar in magnitude to that of traditional risk factors, such as age (18% per 10-year increase) and blood pressure (9% per 10–mm Hg increase).
Our HIV-uninfected population was selected to reflect generally healthy adults with similar sociodemographic characteristics. Comparisons with our severely immunosuppressed HIV-infected population therefore reflect the extremes, and this also limits the generalizability of our study to unselected HIV populations. However, WHO disease stage, CD4+ T-cell count, and presence of coinfections and their clinical markers were not independently associated with arterial stiffness, suggesting that the effects of HIV may exist across the disease spectrum. Acute coinfections at the time of diagnosis were rare, limiting our power to assess their effects, but they may have contributed to an IRIS-type phenomenon. To avoid overburdening potentially clinically unwell patients who urgently needed to start ART, enrollment assessments were performed 2 weeks after ART initiation. As early mortality is high and some limited normalization may have occurred, our findings are likely a best-case scenario [24].
Last, the size of the effect of PD-1+CD8+ T cells on arterial stiffness was modest and is likely to represent one component of several concurrent complex mechanisms involved in the pathogenesis of endothelial damage in people living with HIV in this setting. Nevertheless, exhaustion was the strongest immune predictor of cfPWV on ART, and other immunophenotyping parameters did not have independent effects after adjusting for PD-1 expression.
This is the first time that the dynamics of arterial stiffness in the first few months of ART have been documented longitudinally and related to cellular markers of immune activation in HIV-infected adults. We have confirmed hypertension as the primary CVD risk factor in the region and demonstrated that consequences of immune activation can be reversed irrespective of CD4+ T-cell count recovery, with early benefits for vasculature. Further studies into infection-driven immune activation, as a risk factor for CVD, are warranted, but guidelines for prevention of CVD in HIV-infected individuals are urgently needed and should focus on hypertension reduction and close monitoring for those starting ART at lower CD4+ T-cell counts.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the participating patients and their families; the staff in the ART clinic and Department of Medicine at Queen Elizabeth Central Hospital, Blantyre, Malawi; and the REALITY trial group (Supplementary Materials) for their support with study design and implementation.
Financial support. This work was supported by the Wellcome Trust (grant number 099934/Z/12/A to C. K., strategic award to the MLW Clinical Research Programme, and core support [via MC_UU_12023/23 and MC_UU_12023/26] to the MRC Clinical Trials Unit at UCL). Cipla, Gilead Sciences, ViiV Healthcare/GlaxoSmithKline, and Merck Sharp and Dohme donated drugs for use in the REALITY trial.
Presented in part: CROI, Boston, Massachusetts, March 2017. Abstract 952.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. WHO. Global Health Observatory repository data http://apps.who.int/gho/data/view.main.22100WHO?. Accessed 11 February 2018.
- 2. Lozano R, Naghavi M, Foreman K, et al. . Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Narayan KM, Miotti PG, Anand NP, et al. . HIV and noncommunicable disease comorbidities in the era of antiretroviral therapy: a vital agenda for research in low- and middle-income country settings. J Acquir Immune Defic Syndr (1999) 2014; 67(Suppl 1):S2–7. [DOI] [PubMed] [Google Scholar]
- 4. Althoff KN, Gange SJ.. A critical epidemiological review of cardiovascular disease risk in HIV-infected adults: the importance of the HIV-uninfected comparison group, confounding, and competing risks. [DOI] [PubMed] [Google Scholar]
- 5. Shah ASV, Stelzle D, Lee KK, et al. . Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV. Circulation 2018; 138:1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Longenecker CT, Sullivan C, Baker JV. Immune activation and cardiovascular disease in chronic HIV infection. Curr Opin HIV AIDS 2016; 11:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rajasuriar R, Khoury G, Kamarulzaman A, French MA, Cameron PU, Lewin SR. Persistent immune activation in chronic HIV infection: do any interventions work? AIDS 2013; 27:1199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Margolick JB, Bream JH, Nilles TL, et al. . Relationship between T-cell Responses to CMV, markers of inflammation, and frailty in HIV-uninfected and HIV-infected men in the multicenter AIDS cohort study. J Infect Dis 2018; 218:249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lawn SD, Little F, Bekker LG, et al. . Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. AIDS 2009; 23:335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walker AS, Prendergast AJ, Mugyenyi P, et al. ; DART and ARROW trial teams Mortality in the year following antiretroviral therapy initiation in HIV-infected adults and children in Uganda and Zimbabwe. Clin Infect Dis 2012; 55:1707–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cappuccio FP. Commentary: epidemiological transition, migration, and cardiovascular disease. Int J Epidemiol 2004; 33:387–8. [DOI] [PubMed] [Google Scholar]
- 12. Mathabire Rücker SC, Tayea A, Bitilinyu-Bangoh J, et al. . High rates of hypertension, diabetes, elevated low-density lipoprotein cholesterol, and cardiovascular disease risk factors in HIV-infected patients in Malawi. AIDS 2018; 32:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Bortel LM, Laurent S, Boutouyrie P, et al. ; Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012; 30:445–8. [DOI] [PubMed] [Google Scholar]
- 14. Laurent S, Cockcroft J, Van Bortel L, et al. ; European Network for Non-invasive Investigation of Large Arteries Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–605. [DOI] [PubMed] [Google Scholar]
- 15. Vlachopoulos C, Xaplanteris P, Aboyans V, et al. . The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015; 241:507–32. [DOI] [PubMed] [Google Scholar]
- 16. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 55:1318–27. [DOI] [PubMed] [Google Scholar]
- 17. Mitchell GF, Hwang SJ, Vasan RS, et al. . Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010; 121:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. . Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 2006; 113:657–63. [DOI] [PubMed] [Google Scholar]
- 19. Gleason RL Jr, Caulk AW, Seifu D, et al. . Efavirenz and ritonavir-boosted lopinavir use exhibited elevated markers of atherosclerosis across age groups in people living with HIV in Ethiopia. J Biomech 2016; 49:2584–92. [DOI] [PubMed] [Google Scholar]
- 20. Ngatchou W, Lemogoum D, Ndobo P, et al. . Increased burden and severity of metabolic syndrome and arterial stiffness in treatment-naïve HIV+ patients from Cameroon. Vasc Health Risk Manag 2013; 9:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lazar JM, Wu X, Shi Q, et al. . Arterial wave reflection in HIV-infected and HIV-uninfected Rwandan women. AIDS Res Hum Retroviruses 2009; 25:877–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siedner MJ, Kim JH, Nakku RS, et al. . HIV infection and arterial stiffness among older-adults taking antiretroviral therapy in rural Uganda. AIDS 2016; 30:667–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peterson I, Ming D, Kelly C, et al. . Unstructured treatment interruption: an important risk factor for arterial stiffness in adult Malawian patients with antiretroviral treatment. AIDS 2016; 30:2373–8. [DOI] [PubMed] [Google Scholar]
- 24. Hakim J, Musiime V, Szubert AJ, et al. ; REALITY Trial Team Enhanced Prophylaxis plus Antiretroviral Therapy for Advanced HIV Infection in Africa. N Engl J Med 2017; 377:233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mallewa J, Szubert AJ, Mugyenyi P, et al. ; REALITY trial team Effect of ready-to-use supplementary food on mortality in severely immunocompromised HIV-infected individuals in Africa initiating antiretroviral therapy (REALITY): an open-label, parallel-group, randomised controlled trial. Lancet HIV 2018; 5:e231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kityo C, Szubert AJ, Siika A, et al. ; REALITY trial team Raltegravir-intensified initial antiretroviral therapy in advanced HIV disease in Africa: a randomised controlled trial. PLoS Med 2018; 15:e1002706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Glennie SJ, Sepako E, Mzinza D, et al. . Impaired CD4 T cell memory response to Streptococcus pneumoniae precedes CD4 T cell depletion in HIV-infected Malawian adults. PLoS One 2011; 6:e25610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989; 74:2527–34. [PubMed] [Google Scholar]
- 29. Nyirenda MJ. Non-communicable diseases in sub-Saharan Africa: understanding the drivers of the epidemic to inform intervention strategies. Int Health 2016; 8:157–8. [DOI] [PubMed] [Google Scholar]
- 30. Ataklte F, Erqou S, Kaptoge S, Taye B, Echouffo-Tcheugui JB, Kengne AP. Burden of undiagnosed hypertension in sub-saharan Africa: a systematic review and meta-analysis. Hypertension 2015; 65:291–8. [DOI] [PubMed] [Google Scholar]
- 31. Borkum MS, Heckmann JM, Manning K, et al. . High prevalence of “non-dipping” blood pressure and vascular stiffness in HIV-infected South Africans on antiretrovirals. PLoS One 2017; 12:e0185003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Benjamin LA, Corbett EL, Connor MD, et al. . HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: a case-control study. Neurology 2016; 86:324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bisson GP, Ramchandani R, Miyahara S, et al. ; Adult AIDS Clinical Trials Group A5274 (REMEMBER) Study Team Risk factors for early mortality on antiretroviral therapy in advanced HIV-infected adults. AIDS 2017; 31:2217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hakim J, Musiime V, Szubert AJ, et al. ; REALITY Trial Team Enhanced Prophylaxis plus Antiretroviral Therapy for Advanced HIV Infection in Africa. N Engl J Med 2017; 377:233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sinha A, Ma Y, Scherzer R, et al. . Role of T‐cell dysfunction, inflammation, and coagulation in microvascular disease in HIV. J Am Heart Assoc 2016; 5:e004243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ghiglione Y, Trifone C, Salido J, et al. . PD-1 expression in HIV-specific CD8+ T-cells prior to antiretroviral therapy is associated with HIV persistence. J Acquir Immune Defic Syndr 2019; 80:1- 6. [DOI] [PubMed] [Google Scholar]
- 37. Paiardini M. Immune-based interventions targeting inflammation and viral persistence. Seattle: CROI, 2017. [Google Scholar]
- 38. Sperk M, Domselaar RV, Neogi U. Immune checkpoints as the immune system regulators and potential biomarkers in HIV-1 infection. Int J Mol Sci 2018; 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cockerham LR, Jain V, Sinclair E, et al. . Programmed death-1 expression on CD4⁺ and CD8⁺ T cells in treated and untreated HIV disease. AIDS 2014; 28:1749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Glennie SJ, Nyirenda M, Williams NA, Heyderman RS. Do multiple concurrent infections in African children cause irreversible immunological damage? Immunology 2012; 135:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Glennie SJ, Williams NA, Heyderman RS. Mucosal immunity in resource-limited setting: is the battle ground different? Trends Microbiol 2010; 18:487–93. [DOI] [PubMed] [Google Scholar]
- 42. Schoffelen AF, de Groot E, Tempelman HA, Visseren FL, Hoepelman AI, Barth RE. Carotid intima media thickness in mainly female HIV-infected subjects in rural South Africa: association with cardiovascular but not HIV-related factors. Clin Infect Dis 2015; 61:1606–14. [DOI] [PubMed] [Google Scholar]
- 43. Ben-Shlomo Y, Spears M, Boustred C, et al. . Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014; 63:636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Orlova IA, Nuraliev EY, Yarovaya EB, Ageev FT. Prognostic value of changes in arterial stiffness in men with coronary artery disease. Vasc Health Risk Manag 2010; 6:1015–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Toyama K, Sugiyama S, Oka H, et al. . Combination treatment of rosuvastatin or atorvastatin, with regular exercise improves arterial wall stiffness in patients with coronary artery disease. PLoS One 2012; 7:e41369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boutouyrie P, Tropeano AI, Asmar R, et al. . Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 2002; 39:10–5. [DOI] [PubMed] [Google Scholar]
- 47. Gelpi M, Afzal S, Lundgren J, et al. . Higher risk of abdominal obesity, elevated low-density lipoprotein cholesterol, and hypertriglyceridemia, but not of hypertension, in people living with human immunodeficiency virus (HIV): results from the copenhagen comorbidity in HIV infection study. Clin Infect Dis 2018; 67:579–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.