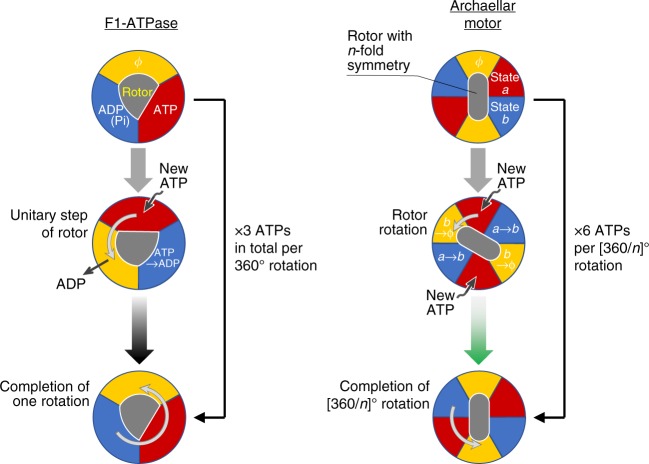
Fig. 4.
A model for the mechanism of ATP hydrolysis by the archaellar motor based on similarity to the F1-ATPase. Left, The mechanism of the F1-ATPase that we have previously demonstrated24–26, 33. Two of the three catalytic subunits are occupied by nucleotides, either ATP (red) or ADP (blue), while the remaining subunit is empty (yellow). In this schematic, the positions of three subunits are fixed while the rotor rotates against them. Because the three catalytic subunits are cooperative, each subunit undergoes a determinate cycle through “empty state (ϕ)” → “ATP binding state” → “post-hydrolysis state” with each discrete 120° rotation. As a consequence, one F1-ATPase with three catalytic subunits consumes three ATPs per rotation, with a single ATP hydrolysis per subunit per 360° rotation. Right, our model for the mechanism of ATP hydrolysis-driven archaellar rotation. The principles are similar to the F1-ATPase, except the catalytic cycle for each catalytic subunit repeats every [360/n]° rotation (green arrow) due to the n-fold symmetry of the rotor and six active sites. Note that the figure is conceptual: our model does not depend on a specific structure and is not intended to be structurally explicit. For simplicity, the schematic is illustrated with the assumption of n = 2, which is consistent with our results and observations of two-fold symmetry in the structure of the cyclic FlaI hexamer8