Abstract
The immune system plays a considerable role in hypertension. In particular, T‐lymphocytes are recognized as important players in its pathogenesis. Despite substantial experimental efforts, the molecular mechanisms underlying the nature of T‐cell activation contributing to an onset of hypertension or disease perpetuation are still elusive. Amongst other cell types, lymphocytes express distinct profiles of GPCRs for sphingosine‐1‐phosphate (S1P) – a bioactive phospholipid that is involved in many critical cell processes and most importantly majorly regulates T‐cell development, lymphocyte recirculation, tissue‐homing patterns and chemotactic responses. Recent findings have revealed a key role for S1P chemotaxis and T‐cell mobilization for the onset of experimental hypertension, and elevated circulating S1P levels have been linked to several inflammation‐associated diseases including hypertension in patients. In this article, we review the recent progress towards understanding how S1P and its receptors regulate immune cell trafficking and function and its potential relevance for the pathophysiology of hypertension.
Linked Articles
This article is part of a themed section on Immune Targets in Hypertension. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.12/issuetoc
Abbreviations
- AngII
angiotensin II
- APC
antigen‐presenting cell
- CCR2
chemokine receptor type 2
- CD
cluster of differentiation
- DCs
dendritic cells
- M1
M1‐polarized macrophages
- M2
M2‐polarized macrophages
- PAH
pulmonary arterial hypertension
- S1P
sphingosine‐1‐phosphate
- S1PL
S1P lyase
- SPHK
sphingosine kinase
- Treg
T regulatory
Sphingosine‐1‐phosphate biosynthesis and degradation
Sphingosine 1‐phosphate (S1P) is a bioactive phospholipid with a complex metabolism typically orchestrated by a number of different enzymes. Two distinct kinases [sphingosine kinase 1 (SPHK1) and SPHK2] generate S1P from sphingosine, which itself is produced by degradation of ceramide (reviewed in Spiegel and Milstien, 2003). Both SPHK isoforms exhibit different catalytic properties, subcellular locations and tissue distribution. While SPHK1 is highly specific for sphingosine and dihydrosphingosine, SPHK2 is able to phosphorylate a broader range of substrates, including sphingosine, dihydrosphingosine, phytosphingosine and fingolimod (reviewed in Spiegel and Milstien, 2003). In contrast to SPHK2 that is mostly found in the nucleus, SPHK1 resides in the cytosol and translocates to the plasma membrane upon activation (Lidington et al., 2009). Most importantly, studies have shown that a fraction of SPHK1 is released to the extracellular space (Ancellin et al., 2002; Rigogliuso et al., 2010), which enables the local production of S1P in the vicinity of its cell‐surface receptors.
Due to its pleiotropic effects, S1P levels need to be tightly controlled. Besides a finely tuned generation, S1P degradation is mediated by three types of enzymes: S1P phosphatase (SPP), S1P lyase (S1PL) and lipid phosphate phosphohydrolase (PPA; also known as LPP). In most cells, S1P is irreversibly degraded by S1PL. SPPs (SPP1 and SPP2), which are highly selective for sphingoid base‐1‐phosphates, dephosphorylate S1P to sphingosine (reviewed in Spiegel and Milstien, 2003). However, due to their intracellular localization, the degradation of extracellular S1P by S1PL and SPPs requires an import mechanism. Several studies have suggested that proteins of the ABC transporter family facilitate this S1P uptake (Boujaoude et al., 2001; Meissner et al., 2012). The only S1P‐catabolizing enzymes with the ability to degrade extracellular S1P belong to the PPA family, which shows broad substrate specificity. In contrast to PPA3A, PPA2A and PPA2B are mainly localized to the plasma membrane and have been shown to degrade extracellular lipid phosphate substrates (reviewed in Spiegel and Milstien, 2003).
Many cell types supply the circulating S1P pool. Amongst them, erythrocytes serve as one of the main S1P sources due to their ability to import sphingosine from the surrounding environment, their lack of S1P degradation enzymes and their constitutive release of S1P to maintain plasma S1P concentrations (reviewed in Thuy et al., 2014). Recently, MFSD2B was characterized as an important S1P transporter in erythroid cells (Vu et al., 2017; Kobayashi et al., 2018). Similarly, platelets possess low levels of active S1PL and can therefore store large amounts of S1P. However, their contribution to physiological homeostasis is minimal since S1P release from platelets is dependent on very specific stimuli, which might support a role for platelet‐mediated S1P release in disease. Like platelets, mast cells release S1P in response to stimuli including allergic reactions. Leukocytes on the other hand only play a minor role in S1P release due to the highly effective S1P‐degrading system in these cells (reviewed in Ksiazek et al., 2015). In addition to haematopoietic cells, cells of non‐haematopoietic origins, such as endothelial cells and hepatocytes, contribute to maintaining homeostatic S1P levels. Endothelial cells provide approximately 40% of the total S1P plasma concentration through exporting S1P via spinster homologue transporter 2 (SPNS2) (Hisano et al., 2012) and by constitutively releasing SPHK1 for extracellular S1P production (Ancellin et al., 2002). S1P release from hepatocytes is still controversial; however, hepatocytes are the major source of S1P carrier apolipoprotein M, which may play a contributory role in maintaining physiological S1P levels (reviewed in Ksiazek et al., 2015).
The tight regulation of S1P levels is vital for the maintenance of S1P gradients between different tissue compartments and thus of significant importance for immune cell trafficking. Because recent investigations suggested a deregulation of S1P levels and its signalling axis during disease, this review commends the importance of S1P signalling in immune cell trafficking and activation and their potential implications in hypertension.
The S1P signalling axis – a busy multitasker
S1P acts as intracellular second messenger as well as an extracellular receptor ligand. Although the intracellular targets of S1P remain largely elusive, a few interesting findings have suggested a role for intracellular S1P in cell‐cycle progression, DNA synthesis and control of apoptosis (reviewed in Payne et al., 2002). S1P's extrinsic functions are mediated via a family of five GPCRs (S1P receptors 1–5). S1P receptors are widely studied entities with important functions in a variety of cells and tissues. With the exception of S1P4 and S1P5 receptors, S1P receptors are essentially ubiquitously expressed. Their expression patterns vary in tissues and change during development and ageing. To date, the S1P1 receptor is the most studied S1P receptor due to its distinct effects in the vasculature and the immune system (Takabe et al., 2008; Maceyka et al., 2009). In the vasculature, endothelial S1P1 receptor activation promotes relaxation of resistance arteries (Cantalupo et al., 2017), increases barrier function or mediates the release of pro‐inflammatory factors from storage granules (Jang et al., 2009). In the immune system, the same receptor fulfils similarly important functions through modulation of lymphocyte trafficking and development (Tarrason et al., 2011). S1P receptor signalling has furthermore revealed a significant involvement in vascular tone regulation through apparent vascular bed‐specific pro‐constrictive function of S1P2 and S1P3 receptors (Hoefer et al., 2010; Yang et al., 2012). Interestingly, both S1P receptors have also been associated with the regulation of myeloid cell migration and activation (Michaud et al., 2010). The S1P3 receptor is further implicated in the regulation of heart rate (Sanna et al., 2004) and, similar to S1P2 receptors, also in vascular permeability (Sanchez et al., 2007). Different S1P receptor‐specific effects are summarized in Figure 1.
Figure 1.
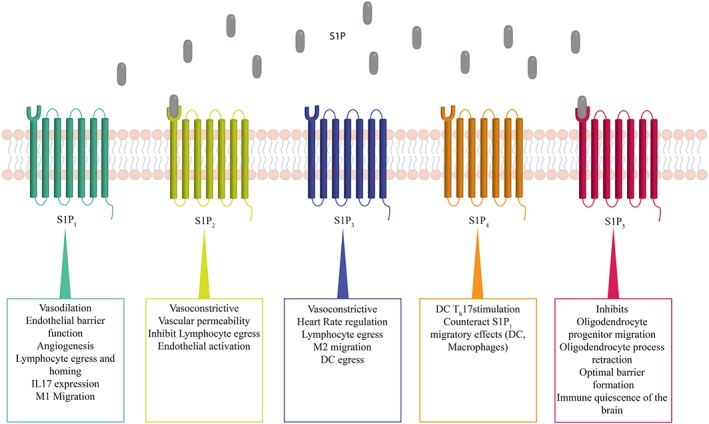
S1P mediates distinct effects via five GPCRs. Extracellular receptor binding facilitates receptor‐specific and cell‐specific effects of S1P. Th17, T helper 17 cells.
Besides the important role of S1P receptors in the regulation of critical vascular and immune cell functions, the involvement of S1P generating enzymes has also been shown: SPHK1 activity modulates Ca2+ sensitivity and thus myogenic responsiveness in resistance arteries (Lidington et al., 2009), critically regulates vascular barrier integrity (Tauseef et al., 2008) and participates in NF‐κB activation (Hait et al., 2009). SPHK2‐mediated effects on the other hand are rather sparsely investigated. Thus far, SPHK2 has been shown to participate in the maintenance of the blood–brain barrier (Wacker et al., 2012), histone acetylation and gene expression (Hait et al., 2009) and the redistribution of blood‐borne S1P into tissues (Sensken et al., 2010).
S1P signalling in hypertension – a doubled‐edged sword
Over the past few years, our understanding of S1P signalling in various cell types and tissues has steadily increased; yet the application of this knowledge to disease is still lagging behind. With respect to hypertension, the information regarding S1P's role in disease development and propagation is similarly sparse. Although several reports indicate enhanced sphingolipid metabolism and increased S1P tissue and plasma concentrations in different forms of experimental and human hypertension (Spijkers et al., 2011; Chen et al., 2014; Tabeling et al., 2015; Zhao et al., 2015; Gairhe et al., 2016; Meissner et al., 2017), the underlying molecular mechanisms are mostly elusive.
In line with that, the expression and activity of several key players of the S1P signalling axis seem to be critically associated with elevated BP levels in different rodent models. In particular, enhanced S1P production through SPHK1 has been linked to unfavourable prognosis in pulmonary arterial hypertension (PAH) in mice and men (Chen et al., 2014). Genetic depletion of SPHK1 protected from the development of experimental PAH (Chen et al., 2014), reduced S1P levels and attenuated the occlusive pulmonary arteriopathy associated with PAH (Gairhe et al., 2016). Interestingly, genetic depletion of the S1P's catabolizing enzyme S1PL promoted hypoxia‐induced hypertension in mice (Chen et al., 2014), pointing towards an important involvement of the S1P signalling axis in PAH. However, exact mechanistic insights are still to be established. Transcriptome profiling recently identified SPHK1 as one of the key modulators of angiotensin II (AngII)‐induced hypertension (Siedlinski et al., 2017). This notion has been strengthened in experimental studies that show blunted BP responses to AngII in mice deficient in SPHK1 (Meissner et al., 2017; Siedlinski et al., 2017). Moreover, genetic network‐based analyses established a central role for components of the ceramide‐S1P rheostat in BP regulation and hypertension (Fenger et al., 2011; Fenger et al., 2015).
Beside S1P production and degradation, S1P receptor activation has been intensively studied with respect to its involvement in the regulation of vascular tone and BP. Several studies showed apparent smooth muscle‐specific effects of S1P2 and S1P3 receptor‐associated signalling (Hoefer et al., 2010; Yang et al., 2012; Cantalupo et al., 2017) where specific S1P2 receptor‐goverened activation of myosin light chain kinase (MLCK) leads to enhancements of myogenic tone in small resistance arteries (Hoefer et al., 2010) and is associated with reduced tissue perfusion (Yang et al., 2012). Cantalupo et al. (2017) assigned S1P3 receptors a prevalent role in vascular smooth muscle‐mediated tone regulation in response to S1P and intraluminal pressure without apparent effects on BP. In the same investigation, the group described an endothelial‐specific offset mechanism to S1P's vasopressor‐mediated signalling through S1P2/3 receptor‐RhoA or S1P2/3 receptor‐MLCK: endothelial S1P‐S1P1 receptor‐NO signalling regulates vascular relaxation in response to flow and hence BP under physiological conditions and in hypertension (Cantalupo et al., 2017). Earlier, the same group identified the endoplasmic reticulum membrane protein reticulon (Nogo‐B) as an important negative regulator of endothelial sphingolipid biosynthesis that critically affects vascular function and BP through an autocrine S1P1 receptor‐NO signalling loop (Cantalupo et al., 2015). Recently, an engineered S1P chaperone (apolipoprotein M‐Fc) was shown to selectively activate the same S1P receptor in the endothelium and thereby attenuated hypertension (Swendeman et al., 2017). Besides its vascular‐specific effects, S1P1 receptor surface expression on T‐cells critically modulates their migration along the S1P gradient (Matloubian et al., 2004) between tissue and blood and is responsible for lymphocyte egress and homing (Rivera et al., 2008). Recent findings also revealed a key role for SPHK2‐driven S1P chemotaxis and T‐cell mobilization for the onset of AngII‐induced hypertension in mice (Meissner et al., 2017).
S1P – conductor in immune cell trafficking
Chemotaxis represents a critical mechanism for proper immune responses, which is influenced by a number of signalling molecules. Amongst them, S1P gradients play a major role in determining egress and homing of immune cells (Rivera et al., 2008). To date, the exact molecular mechanisms underlying tissue‐specific S1P concentrations and thus the development of distinct S1P gradients are not very well understood. However, the regulation of the S1P gradient seems to involve a specific spatial contribution of different S1P signalling components: while SPHKs synthesize S1P in lymphatic endothelium (Pham et al., 2010), in platelets (Fukuda et al., 2003; Urtz et al., 2015) and red blood cells (Ochi et al., 2004), S1P‐catabolizing enzymes such as S1PL, SPPs and PPAs control S1P levels within the primary and secondary lymphoid organs (Schwab et al., 2005; Breart et al., 2011; Park et al., 2014). In the thymus, the PPA2B‐mediated regulation of S1P levels critically controls the S1P gradient and thus thymic egress (Breart et al., 2011). Furthermore, the involvement of sphingolipid transporters (SPNS) in the maintenance of S1P gradients has been reported. In particular, SPNS2 was shown to mediate the release of S1P from the vascular endothelium rather than platelets or red blood cells (Fukuhara et al., 2012; Hisano et al., 2012). Mice deficient in SPNS2 exhibit reduced S1P plasma levels and lymphopaenia, suggesting a vital role for SPNS in lymphocyte egress from the thymus (Fukuhara et al., 2012; Hisano et al., 2012). Hisano et al. (2012) furthermore conclude that SPNS2 deficiency has no effect on secondary lymphoid organ S1P concentrations. However, in the lymph of these animals, S1P levels are severely affected by SPNS2 depletion with implications for lymphocyte egress from lymph nodes and hence the number of circulating lymphocytes (Mendoza et al., 2012). The same group recently described a critical role for S1P, secreted from lymphatic endothelial cells by a SPNS2‐dependent mechanism, in S1P1 receptor‐mediated naïve T‐cell viability (Mendoza et al., 2017).
Additionally, different S1P chaperones are thought to deliver different biological functions of S1P: unlike albumin‐bound S1P, S1P bound to apolipoprotein M restrains lymphopoiesis in the bone marrow and suppresses lymphocyte progenitor generation via S1P1 receptor activation (Blaho et al., 2015).
Of significance to S1P‐governed chemotaxis is the expression of specific S1P receptors on immune cells. Different subtypes of immune cells rely on different S1P receptors to migrate towards higher S1P concentrations. In the innate immune system, a reliance on all of the S1P receptors besides S1P5 has previously been demonstrated (Maeda et al., 2007; Schulze et al., 2011; Awojoodu et al., 2013; Weichand et al., 2013), while cells of the adaptive immune system require mostly S1P1 receptors to migrate along the S1P gradient (reviewed in Park and Im, 2017). T‐cell trafficking along the S1P gradient has been of significant interest to scientists and the pharmaceutical industry. The ability of T‐cells to migrate towards S1P has been successfully exploited for clinical applications in multiple sclerosis therapy (fingolimod). Fingolimod induces lymphopaenia via agonistic activation and subsequent internalization of S1P1 receptors on lymphocytes. A deficiency in S1P1 receptor surface expression blocks lymphocytes egress from secondary lymphoid organs along the S1P gradient (reviewed in Park and Im, 2017). Most T‐cells down‐regulate their surface S1P1 receptor S1PR1 expression upon activation and hence lose their chemotactic response to S1P. This in turn can increase their responsiveness to chemokines responsible for T‐cell homing to the spleen or lymph node. In line with that, a permanent down‐regulation of S1P1 receptors is required for the establishment of tissue‐resident memory T‐cells (Skon et al., 2013).
S1P‐governed trafficking is somewhat more complex for cells of the myeloid lineage [dendritic cells (DCs) and monocytes/macrophages] as they are typically tissue residents and only upon activation do they migrate into the circulation.Specifically, their responsiveness to S1P is regulated by activation‐associated S1P receptor expression. Prior to maturation, DCs mainly express S1P2 receptors, which is thought to counteract the pro‐migratory role of S1P1 receptors. During maturation, the expression of S1P1 and S1P3 receptors is stimulated, thus enabling DC egress into the lymphatic system (Czeloth et al., 2005). Likewise, macrophages require specific S1P receptor expression patterns to facilitate S1P‐guided migration to and from sites of inflammation (Weichand et al., 2013).
Despite the recent advancements in the field, further research is needed to underpin the current concepts and to understand the molecular regulators of pathogenic S1P shifts and S1P‐governed immune cell trafficking. In hypertension research, evidence has accumulated showing elevated levels of circulating and tissue‐specific S1P (Spijkers et al., 2011; Chen et al., 2014; Tabeling et al., 2015; Zhao et al., 2015; Gairhe et al., 2016; Meissner et al., 2017), which posits S1P chemotaxis as a potential link between inflammation and high BP.
S1P signalling in adaptive and innate immune responses
Adaptive immunity
Aberrant activation of adaptive immunity appears to play a key role in the pathogenesis of hypertension; conversely, its suppression has been shown to attenuate experimental and human hypertension. Despite numerous research efforts, the exact mechanisms underlying the activation of adaptive immune responses during hypertension are still elusive. A growing body of evidence demonstrating S1P's apparent role in immune cell trafficking, T‐cell differentiation and cytokine production positions S1P signalling as an important player in the control of immune responses that might well be critical in the pathogenesis of hypertension. The following section describes S1P involvement in adaptive immune responses of relevance to hypertension.
T‐lymphocytes
In various experimental models, T‐cell mobilization and activation crucially promote the development of sustained hypertension in a feedforward fashion (Marvar et al., 2010; Meissner et al., 2017). T‐cell invasion in critical end‐organ tissues in response to hypertension was shown in various rodent models as well as humanized mouse models (Guzik et al., 2007; Itani et al., 2016; Meissner et al., 2017). A major determinant for T‐cell mobilization is the existing S1P gradient between tissue and blood that drives lymphocyte egress and homing (Rivera et al., 2008) and is governed by S1P1 receptor surface expression on T‐cells (Matloubian et al., 2004). This gradient is devoid in conditional‐knockout mice that lack both S1P‐generating enzymes; consequently, T‐cell exit from lymphoid organs is absent in these mice (Pappu et al., 2007). Recently, our group showed that haematopoietic SPHK2 activity majorly contributes to a greater S1P gradient between blood and lymphoid tissue in AngII‐induced hypertension (Meissner et al., 2017). Systemic as well as haematopoietic depletion of SPHK2 protected from AngII‐induced elevation of BP by preventing increases in plasma S1P and thus diminishing S1P‐driven T‐cell migration into the blood stream. At the same time, blocking the development of a sufficient S1P gradient responsible for the augmented T‐cell egress in response to AngII led to the accumulation of T‐cells in lymphoid tissue (concept is illustrated in Figure 2). Our data furthermore support the possibility that chronic AngII treatment not only enhances T‐cell mobilization from secondary lymphoid organs but also regulates T‐cell trafficking from blood to the lymphatic tissue (Meissner et al., 2017). Although its involvement in BP regulation has not been demonstrated yet, the inhibition of S1P's catabolizing enzyme S1PL results in an increase in thymus, spleen and lymph node S1P concentrations, thus causing a loss of circulating lymphocytes (Schwab et al., 2005; Vogel et al., 2009; Weiler et al., 2014). Interestingly, interfering with S1P catabolism showed no effect on plasma S1P concentrations (Schwab et al., 2005; Harris et al., 2016). As previously mentioned, the spatial contribution of different S1P signalling components to creating S1P gradients is highly dependent on tissue and cell type. Although a growing body of publications has generated initial basic mechanistic insight into this complex system with respect to immune cell trafficking (Schwab et al., 2005; Breart et al., 2011; Fukuhara et al., 2012; Hisano et al., 2012; Mendoza et al., 2012; Harris et al., 2016), hypertension‐associated shifts in compartment‐specific S1P levels and associated immune cell function still need to be elucidated.
Figure 2.
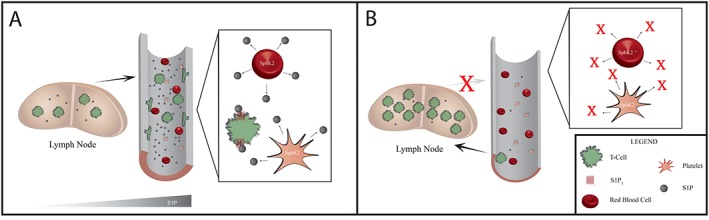
SPHK2‐mediated T‐cell trafficking in AngII‐induced hypertension according to Meissner et al. (2017). (A) The SPHK2‐mediated increase in plasma S1P levels governs T‐cell mobilization from secondary lymphoid tissue in AngII‐induced hypertension. S1P further facilitates endothelial activation and the expression of molecules necessary for T‐cell adhesion. The resulting infiltration and accumulation of T‐cells within the vascular wall critically alters vascular structure and function. (B) The inhibition of SPHK2 in haematopoietic cells prevents the AngII‐induced S1P generation. The lack of S1P response flattens the S1P gradient and hinders T‐cells egress into the blood in response to AngII. Subsequently, T‐cells accumulate within the secondary lymphoid tissue.
The synthetic S1P receptor modulator fingolimod circumvents the S1P gradient by antagonizing S1P1 receptors on T‐lymphocytes. By triggering the internalization of the S1P sensor in T‐cells, fingolimod inhibits T‐cell egress from lymphoid tissue and the recruitment of T‐cells to sites of local inflammation (Mandala et al., 2002). Fingolimod has proven efficacy in preventing the onset of experimental hypertension; however, its administration at times of already established hypertension failed to reduce BP levels despite inducing profound lymphopaenia (Meissner et al., 2017). This may be due to its inability to reduce the number of memory T‐cells (Hofmann et al., 2006) or to its disadvantageous effects on endothelial NO production (Cantalupo et al., 2017) with deleterious consequences for tissue inflammation and vascular function, respectively. The loss of S1P1 receptor surface expression on mature lymphocytes has also been described after inhibition of S1PL using the food colourant 2‐acetyl‐4‐tetrahydroxylbutylimidazole (THI). This might result from the elevation of S1P concentration in lymphatic organs observed after THI‐induced S1PL inhibition (Schwab et al., 2005). Together, this might account for the defective lymphocyte egress from lymphoid organs after THI treatment (Schwab et al., 2005).
With respect to hypertension, T‐cells are the most studied immune cells. Still, most underlying mechanisms contributing to disease onset or progression remain elusive. Emerging evidence supporting a critical involvement of S1P signalling in T‐cell homeostasis encourages further research to achieve satisfying answers to burning open questions. In particular, future research needs to address the nature of S1P gradients between lymphoid and non‐lymphoid organs and how they change during hypertension, or how T‐cell‐specific S1P receptor activity promotes T‐cell viability, egress and tissue retention. In line with this, wherther the modulation of S1P1 receptors impacts immunity either directly through signalling pathways or secondary to trafficking‐dependent effects needs to be determined.
Th17 cells
In hypertensive patients, the increased percentage of circulating T‐cells has been linked to the production of large amounts of pro‐inflammatory cytokines (Youn et al., 2013; Itani et al., 2016). Elevated circulating IL‐17A producing CD4+ T‐cells as observed in hypertensive patients (Itani et al., 2016) might critically contribute to the exacerbation of tissue damage and disease progression. In murine models, genetic deletion of IL‐17A as well as the administration of monoclonal antibodies to IL‐17A has been shown to protect from hypertension (Madhur et al., 2010), while the infusion of recombinant IL‐17A elevated BP (Nguyen et al., 2013).
Remarkably, S1P is suggested to be involved in augmented Th17 cell development, migration and IL‐17 generation (Liao et al., 2007; Garris et al., 2013; Eken et al., 2017). The mitigation of IL‐17 generation mediated by the unspecific S1P receptor modulator fingolimod implicates substantial involvement of S1P receptor activation in these processes (Liao et al., 2007; Maeda et al., 2015). Experimental approaches specifically targeting S1P1 receptors revealed an important role for this particular S1P receptor; S1P1 receptor agonism significantly augmented IL‐17 generation by CD4+ T‐cells (Liao et al., 2007). A direct involvement of the S1P1 receptor in IL‐17 generation is furthermore supported by findings that show elevated IL‐17 production in primary T‐cell or antigen‐presenting cell (APC) cultures isolated from mice expressing S1P1 receptors with a phosphorylation deficiency in a residue that is crucial for receptor internalization (Garris et al., 2013). The researchers concluded that impaired S1P1 receptor phosphorylation and thus compromised S1P1 receptor internalization enhances Th17 polarization and tissue infiltration, which might also bear significance in the pathogenesis of hypertension. Th17‐specific S1P1 receptor depletion indicated proliferation and differentiation defects as well as an involvement of the S1P1 receptor in Th17 distribution (Eken et al., 2017). The same report indicates an accumulation of IL‐17A producing CD4+ cells in lymph nodes after acute S1P1 receptor deletion, suggesting the involvement of S1P1 receptors not only in the generation and peripheral distribution of Th17 cells but also in Th17 cell homing to peripheral organs. Interestingly, the same group presented ongoing work where they used specific S1P1 receptor agonism to inhibit human Th17 development from naïve T‐cells and IL‐17 production ex vivo. It remains to be determined whether blocking the generation of APC‐derived cytokines is involved in Th17 polarization of T‐cells, or direct effects on T‐cells might be causative to this inhibition.
Although experimental evidence supports an important involvement of the S1P‐ S1P1 receptor axis in Th17 development, polarization and migration and its contribution to tissue inflammation, the connection to Th17‐driven immunity in hypertension is yet to be determined. Th17 priming is further complicated by evidence that beside S1Ps direct action, multiple immune cell subtypes, possibly via S1P, contribute to Th17 activation. An elucidation of the exact mechanisms involved in increased Th17 signalling in different models of hypertension is therefore needed. In this context, the specific elucidation of S1P1 receptor‐related signalling leading to Th17 priming would be of particular importance.
Regulatory T‐cells
T regulatory (Treg) cells represent an important immune suppressor subtype that is thought to be critical for the regulation of arterial BP. Decreases in Treg populations have been associated with the pathogenesis of hypertension in several experimental models (Barhoumi et al., 2011; Mian et al., 2016). In line with this, adoptive transfer of Tregs isolated from normotensive mice reduced AngII‐induced BP levels (Barhoumi et al., 2011). Recently, an elegant study showed that forkhead box P3 (FOXP3)‐deficient Tregs exacerbates AngII‐induced hypertension by enhancing innate and adaptive immune responses (Mian et al., 2016).
The role of S1P and specifically that of S1P1 receptor activation seems most controversial in this specific T‐cell subset. Differentiation of pro‐inflammatory Th1 cells and anti‐inflammatory Treg cells was reported to be reciprocally regulated by S1P1 receptor signalling (Liu et al., 2010). While deletion of S1P1 receptors in T‐cells using a CD4cre system significantly improved Treg generation and function, S1P1 receptor overexpression in CD4+ T‐cells reduced their differentiation into Treg cells (Liu et al., 2009). Interestingly, the Treg cells found in S1P1 receptor‐deficient mice revealed an activated phenotype and were more prone to apoptosis, thus converted to effector Treg (Eken et al., 2017). Thus, S1P1 receptors have been shown to not only regulate phenotypic diversity but also Treg egress cells from lymphoid organs and subsequent non‐lymphoid tissue distribution (Eken et al., 2017). These somewhat contradictory findings raise more questions than answers regarding the role of the S1P signalling axis during Treg‐mediated inflammatory responses, necessitating further investigation. Of particular interest in this context would be the elucidation of S1P1 receptor‐dependent Treg cell generation in the thymus and its role in their distribution to secondary lymphoid organs. Moreover, potential S1P1 receptor‐mediated migratory properties of Treg cells to non‐lymphoid tissue might be of significance in the evaluation of treatment options involving the modulation of S1P receptors for hypertension and associated target organ damage.
B‐lymphocytes
In both experimental and human hypertension, the elevation of several antibodies produced by B‐cells has been reported (Chan et al., 2014; Chan et al., 2015). Because B‐cell activation can be dependent or independent of T‐cells, the absence of an AngII response in Rag1‐deficient mice after adoptive B‐cell transfer is not surprising (Guzik et al., 2007). Recently, experimental evidence revealed that a genetic deficiency of B‐cell‐activating factor receptors, or pharmacological depletion of B‐cells, protects against the BP elevation and organ damage induced by AngII (Chan et al., 2015).
Similar to T‐lymphocytes, B‐cells migrate along the S1P gradient (Pereira et al., 2010). Like in T‐cells, the S1P1 receptor promotes marginal zone B‐cell migration (Pereira et al., 2010) towards the bone marrow vascular compartment and the peripheral blood. Comparable with T‐cells (Shiow et al., 2006), this S1P1 receptor‐mediated egress is inhibited by the expression of CD69 (Sic et al., 2014). In contrast to T‐cells, the activation of S1P2 and S1P4 receptors in B‐lymphocytes also reduces S1P1 receptor‐mediated B‐cell migration (Sic et al., 2014). However, most interestingly and of potential significance for the pathology of hypertension is the finding that B‐cells migrated towards elevated pulmonary S1P concentrations in the inflamed lung of S1P‐treated mice and these S1P stimulated B‐cells release TNF‐α and granzyme B (Sorrentino et al., 2015).
Future research needs to address the open questions regarding the role of B‐cells in hypertension and the potential involvement of S1P‐governed B‐cell distributions. Specifically, an increase of knowledge regarding a potential role of the S1P signalling axis in T‐cell dependent B‐cell activation might provide valuable insights into hypertension onset and maintenance.
Innate immunity
Several experimental and clinical studies have tackled the involvement of adaptive immunity in respect to the pathophysiology of hypertension; however, the role of innate immune responses during hypertension is not completely understood. Besides adaptive immune responses, innate immunity may be an important mediator of chronic inflammation in hypertension, which can be facilitated by DCs and monocytes/macrophages. During hypertension, the innate immune system contributes to inflammation either directly or indirectly by activating adaptive immune responses mediated mainly by T‐lymphocytes. Several studies have identified an essential role for S1P and its receptors in a variety of innate immune responses.
Dendritic cells
DCs represent a major class of APCs that form a critical link between the adaptive and innate immune system. Although DC function in hypertension is not yet clearly understood, experimental evidence suggests a critical role for DCs in the stimulation of hypertension‐associated inflammation, as the inhibition of DC maturation blunted BP responses to AngII (Vinh et al., 2010). Likewise, the transfer of mature DCs from hypertensive into normotensive mice was associated with increased activation of T‐cells and hypertension (Kirabo et al., 2014).
Due to the relatively sparse knowledge regarding the role of DCs in the pathogenesis of hypertension, investigations exploring the involvement of DC‐related S1P signalling in hypertension are lacking. However, various reports show a close connection between S1P signalling and DC chemotaxis, cytokine production and maturation, which may have implications in hypertension. Besides mediating DC trafficking along an S1P gradient, S1P receptor expression has been implicated in the regulation of DC maturation and cytokine production: DC‐specific depletion of S1P4 receptors critically reduced Th17 differentiation and shifted immune responses towards Th2‐dominated responses (Schulze et al., 2011). Similarly, S1P1‐deficient and S1P3‐deficient DCs promoted a shift towards an anti‐inflammatory environment and Th2/IL‐4 responses respectively (Bajwa et al., 2012). DC‐specific S1P4 receptor‐dependent cytokine production appears to be most influential during chronic inflammation as it can promote for instance, Th17 differentiation of T‐cells. This may render the S1P4 receptor in DCs an attractive target in chronic inflammatory disease.
The modulation of S1P degradation has also been suggested to play a role in DC‐associated inflammation. Specific genetic deletion of S1PL in DCs was linked to impaired T‐cell egress from the thymus, pointing to a significant involvement of DCs in the generation of S1P gradients and thus the homeostatic regulation of thymic T‐cell export (Zamora‐Pineda et al., 2016).
DC dependence on S1P is still not clearly understood, and there remains some disagreement as to which S1P receptors are involved in pro‐inflammatory versus anti‐inflammatory effects. Nonetheless, DC‐associated S1P signalling might present a promising target to blunting the immune response during hypertension. In particular, understanding the role of S1P‐S1P receptor signalling in Th17 and Th2 polarization might prove important in hypertension‐associated immune responses. The notion that DCs potentially contribute to the generation of S1P gradients could furthermore strengthen the link between innate and adaptive immune responses during the pathogenesis of hypertension.
Monocytes and macrophages
Monocytes and macrophages have been shown to play a role in various experimental models as well as in human hypertension (Chan et al., 2012; Moore et al., 2015; Nosalski et al., 2017). During hypertension, macrophages and their circulating precursor monocytes preferentially accumulate in the vasculature, the heart and the kidneys (Chan et al., 2012). The release of pro‐inflammatory mediators such as monocyte chemotactic protein 1 (MCP‐1/CCL2) , C‐C chemokine receptor type 2 (CCR2) and free radicals by infiltrating macrophages drastically affects vascular function and augments hypertension. Respectively, leukocyte‐selective CCR2 deficiency and pharmacological blockade of macrophage CCR2 receptors abolished AngII‐associated inflammation, vascular remodelling and blunted BP responses (Chan et al., 2012; Moore et al., 2015).
With respect to monocyte/macrophage populations, the role of S1P has mainly been studied in the vascular endothelium where S1P‐S1P receptor‐mediated endothelial activation increases the adhesion of monocytes. Here, endothelial‐specific S1P receptor modulation for instance S1P1 receptor agonism critically reduced monocytes’ interaction with the endothelial cell layer. Additionally, several investigations also confirmed a critical role for monocyte/macrophage‐specific S1P receptor expression in the regulation of their migration and activation. In general, monocytes’ migration is mainly mediated through S1P1 and S1P3 receptors (Yang et al., 2015), while the S1P2 receptor acts as a negative regulator of monocyte migration (Michaud et al., 2010). Macrophage migration along the S1P gradient seems to be subtype‐dependent with apparent S1P receptor specificity: while the anti‐inflammatory M2 migration is driven by S1P3 receptors (Awojoodu et al., 2013), the migration of pro‐inflammatory M1 is mediated via S1P1 receptors (Weichand et al., 2013). S1P1 receptor, highly expressed on naïve macrophages, is decreased in both M1‐ and M2‐polarized macrophages, while S1P4 receptor expression is only reduced in M1‐polarized cells. Because their migration potential was shown to be higher compared with M2 macrophages, it has been suggested that the ratio between S1P1/S1P4 receptors orchestrates macrophage migration (Muller et al., 2017).
Controversial findings have painted a blurry picture of S1P receptor involvement in monocyte/macrophage activation and chemotaxis. While fingolimod treatment resulted in a reduction of circulating monocytes levels, the same modulator reduces monocyte activation and egress from secondary lymphoid tissue (Lewis et al., 2013) and enhanced the recruitment of anti‐inflammatory, pro‐angiogenic monocytes (Awojoodu et al., 2013). Further work is needed not only to elucidate the role of S1P in monocytes/macrophages activation and migration but also to dissect their contribution to the pathophysiology of hypertension. Specifically, elucidation of the involvement of S1P signalling in monocyte differentiation, cytokine and chemokine expression and the generation of ROS from activated monocytes/macrophages might help to generate novel insights to immune system activation in hypertension and associated target organ damage. Moreover, further in vivo experiments specifically targeting the different S1P receptors are required to corroborate the physiological relevance of the differential S1P profiles in macrophage populations in health and disease.
Inflammasome activation
Experimental evidence strongly implicates an involvement of inflammasome activation in the initiation or progression of chronic diseases, including hypertension (Krishnan et al., 2016). Inflammasomes are multimeric protein complexes that orchestrate the cleavage of the pro‐inflammatory IL‐1β or IL‐18. After sensing pathogen‐associated molecular patterns or danger‐associated molecular patterns, the inflammasome complex recruits pro‐caspase 1, which after auto‐cleavage converts inactive pro‐ILs into their mature forms. Currently, the most studied inflammasome is the NOD‐like receptor family pyrin domain‐containing protein (NLRP3) complex. Blocking NLRP3 assembly protected from DOCA salt‐induced hypertension (Krishnan et al., 2016). Similarly, a NLRP3 deficiency prevents BP responses to AngII (Shirasuna et al., 2015).
Due to the scarcity of mechanistic studies regarding the role of inflammasome activation during hypertension, not much is known about a potential involvement of S1P signalling in hypertension‐related inflammasome activation. Nonetheless, exciting studies showing a NLRP3‐dependent production of IL‐1β in response to sphingosine and after SPHK1 activation in differentiated macrophages (Barbour et al., 2017) suggest that S1P could also be an interesting target in hypertension‐associated inflammasome activation. Particularly, macrophage surface expression of S1P1 receptors evolves as a key mediator in NLRP3 activation and hence IL‐1β generation: in S1P1 receptor‐deficient mice, NLRP3 expression and associated IL‐1β production is significantly reduced in tumour‐associated macrophages. Likewise, caspase‐1 inhibition resulted in a decrease of S1P1 receptor surface expression (Barbour et al., 2017). These exciting findings might stimulate further research to establishing a link between inflammasome activation and S1P signalling in hypertension.
Targeting S1P signalling in hypertension
The mounting evidence showing important immune‐modulatory functions for S1P has spurred an interest in identifying pharmacological targets within its signalling axis. Currently, several selective S1P receptor modulators are being subjected to clinical trials, but only one rather unspecific modulator has been approved for clinically use: fingolimod reduces lymphocyte egress from secondary lymphoid organ, thymus and bone marrow and thus induces lymphopaenia. In vivo, fingolimod is phosphorylated by SPHK2 and modulates four out of five S1P receptors (S1P1, S1P3, S1P4 and S1P5). While acting mainly as an agonist, chronic fingolimod treatment leads to initial S1P1 receptor internalization and eventual degradation of the receptor. In hypertension, fingolimod has been shown to protect from AngII‐induced hypertension when preventatively administered but failed to reduce already established hypertension (Meissner et al., 2017). Similarly, fingolimod exerted BP‐lowering properties in an acute setting likely mediated through the activation of the S1P1 receptor‐NO pathway (Cantalupo et al., 2015). Chronic fingolimod treatment, however, drastically exacerbated hypertension (Spijkers et al., 2012; Cantalupo et al., 2017; Meissner, 2017). Due to reported adverse side effects exerted by fingolimod, the focus has shifted towards developing more specific S1P receptor modulators. Similar to fingolimod, the specific S1P1 agonist SEW2871 showed efficacy in lowering circulating lymphocyte levels (Park and Im, 2017) and BP in an acute setting (Cantalupo et al., 2015; Cantalupo et al., 2017). Besides S1P1 receptors, emerging evidence suggests that the other four S1P receptors are similarly viable targets for modulating immune responses and BP. A large number of S1P receptor agonists and antagonists are currently being explored; an overview of their effects on immunity and BP is summarized in Table 1.
Table 1.
Summary of drugs targeting the S1P pathway and their known effects in hypertension and on the immune system
| Drug | Target (function) | Effect in hypertension | Effect on immune system |
|---|---|---|---|
| FTY720 (fingolimod) | S1P1 R (agonist, functional antagonist) |
Acute: reduction in BP Chronic: elevated BP |
Induces lymphopaenia, phosphorylated by SPHK2, and leads to internalization and degradation of S1P1 receptors |
| SEW2871 | S1P1 R (agonist) | Reduces BP | Induces lymphopaenia by S1P1 R internalization |
| KRP‐203 | S1P1 and S1P5 R (agonist) | No effect on BP | Regulates lymphocyte homing, inhibits macrophage and CD4+ T‐cell migration, inhibits T‐cell activation, decreases T‐cell proliferation and IL‐2 and IFN‐γ production in splenocytes and reduces macrophage expression of activation markers |
| AUY954 | S1P1 R (agonist) | NT | Suppresses infiltration of T‐cells, B‐cell and macrophages in sciatic nerves in EAN |
| APD334 | S1P1 R (agonist) | NT | Lowers the number of absolute number of B‐cells, NK cells and T‐cells |
| BAF312 | S1P1 and S1P5 R (agonist) | No effect on BP | Induces lymphopaenia and reduces T‐cell and B‐cell numbers in blood of healthy individuals in 4–6 h |
| VPC23153 | S1P1 and S1P4 R (agonist) | Enhanced contraction of isolated pulmonary arteries | NT |
| NIBR‐0213 | S1P1 R (antagonist) | NT | Reduces peripheral blood lymphocytes and induces expression of CD69 |
| W146 | S1P1 R (antagonist) | Reduces vasodilatation | Induces lymphopaenia via internalization and degradation of the S1PR1 and decreases the number of CD4+, CD8+ and CD19+ cells in the blood |
| JTE013 | S1P2 R (antagonist) | Decreased risk of HPH | Reduces pro‐inflammatory cytokines via inhibition of CCL3 production |
| SKI2 | SPHK1 and S1PR2 (inhibitor) | Prevented HPH | Blocks neutrophil‐induced endothelial cell permeability |
| THI | S1P lyase inhibitor | NT | Induces lymphopaenia by disrupting the S1P gradient between lymphoid organs and blood |
| ABC294640 | SPHK2 (inhibitor) | Reduces BP | Inhibits NF‐κB, STAT3 and AKT activation |
| K145 | SPHK2 (inhibitor) | Reduces BP | Shows efficacy against myeloma and lymphoma cell lines, leading to cell death |
| SLP7111228 | SPHK1 (inhibitor) | Decreases plasma S1P and decreases occlusive lesions in PAH | Decreases level of S1P in histiocytic lymphoma cells |
| PF‐543 | SPHK1 (inhibitor) | Abolished AngII‐induced mesenteric artery contraction | Increases CD4+ T‐cell, monocyte and macrophage in blood |
R, receptor; CCL3, chemokine (C–C motif) ligand 3; EAN, experimental autoimmune neuritis; HPH, hypoxia‐induced pulmonary hypertension; NT, not tested.
In addition to the S1P receptors, drugs targeting S1P generating enzymes have been developed and tested in various experimental models. SPHK1 inhibition using SLP7111228 showed an overall lowering of plasma S1P levels and lower occurrence of occlusive arteriopathy but failed to reduce BP in PAH (Gairhe et al., 2016). While the SPHK1 inhibitor DMS failed to reduce elevated BP in a murine model of AngII‐induced hypertension, the SPHK2‐specific inhibitors ABC294640 and K‐145 diminished hypertension in these mice (Meissner et al., 2017). Of note, the SPHK2‐specific inhibitor ABC294640 has successfully undergone phase 1 clinical trials for treatment of solid tumours.
Recently, the food colourant THI was discovered to cause lymphopaenia due to its potent inhibitory effects on the S1P‐degrading enzyme S1PL (Schwab et al., 2005). In vitro studies have had difficulties replicating this inhibitory effect, which spurred the interest in developing better‐understood S1PL inhibitors. Specifically, findings that show the importance of S1PL for normal development suggest that long term targeting of S1PL may be toxic (Kumar et al., 2017).
Due to S1P's pleiotropic effect and the proven involvement in immune cell regulation and vascular function, the S1P pathway presents a viable target for a multitude of diseases. But problems regarding specificity, toxicity and understanding of the mechanism of actions are hindering current drug development. Prior to successful exploitation of S1P signalling modulation in disease, a better understanding of the role of the S1P pathway in disease progression is needed.
Concluding remarks and future directions
Central to the exploitation of S1P signalling as an immune target in hypertension is the elucidation of how the diversity of S1P‐mediated effects may be integrated into a coordinated immune response. The key to resolving this is an expansion of our mechanistic understanding about the actual role of specific immune cells in hypertension, how S1P levels control the function of various immune cells beyond trafficking and which of the diverse effects of S1P on the immune system are relevant to the pathogenesis of hypertension. Above all, further investigations concerning the role of S1P signalling in immune‐cell differentiation might help to isolate more specific therapeutic targets.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a, 2017b, 2017c).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors acknowledge the following funding sources: the Knut and Alice Wallenberg Foundation for generous support, the Swedish Research Council (VR; 2017‐01243), the German Research Foundation (DFG; ME 4667/2‐1) and the Åke Wibergs Stiftelse (M17‐0031).
Don‐Doncow N., Zhang Y., Matuskova H., and Meissner A. (2019) The emerging alliance of sphingosine‐1‐phosphate signalling and immune cells: from basic mechanisms to implications in hypertension, British Journal of Pharmacology, 176, 1989–2001, doi: 10.1111/bph.14381.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. Br J Pharmacol 174: S225–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS et al (2002). Extracellular export of sphingosine kinase‐1 enzyme. Sphingosine 1‐phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem 277: 6667–6675. [DOI] [PubMed] [Google Scholar]
- Awojoodu AO, Ogle ME, Sefcik LS, Bowers DT, Martin K, Brayman KL et al (2013). Sphingosine 1‐phosphate receptor 3 regulates recruitment of anti‐inflammatory monocytes to microvessels during implant arteriogenesis. Proc Natl Acad Sci U S A 110: 13785–13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajwa A, Huang L, Ye H, Dondeti K, Song S, Rosin DL et al (2012). Dendritic cell sphingosine 1‐phosphate receptor‐3 regulates Th1‐Th2 polarity in kidney ischemia–reperfusion injury. J Immunol 189: 2584–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour M, McNaughton M, Boomkamp SD, MacRitchie N, Jiang HR, Pyne NJ et al (2017). Effect of sphingosine kinase modulators on interleukin‐1β release, sphingosine 1‐phosphate receptor 1 expression and experimental autoimmune encephalomyelitis. Br J Pharmacol 174: 210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF et al (2011). T regulatory lymphocytes prevent angiotensin II‐induced hypertension and vascular injury. Hypertension 57: 469–476. [DOI] [PubMed] [Google Scholar]
- Blaho VA, Galvani S, Engelbrecht E, Liu C, Swendeman SL, Kono M et al (2015). HDL‐bound sphingosine‐1‐phosphate restrains lymphopoiesis and neuroinflammation. Nature 523: 342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boujaoude LC, Bradshaw‐Wilder C, Mao C, Cohn J, Ogretmen B, Hannun YA et al (2001). Cystic fibrosis transmembrane regulator regulates uptake of sphingoid base phosphates and lysophosphatidic acid: modulation of cellular activity of sphingosine 1‐phosphate. J Biol Chem 276: 35258–35264. [DOI] [PubMed] [Google Scholar]
- Breart B, Ramos‐Perez WD, Mendoza A, Salous AK, Gobert M, Huang Y et al (2011). Lipid phosphate phosphatase 3 enables efficient thymic egress. J Exp Med 208: 1267–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo A, Gargiulo A, Dautaj E, Liu C, Zhang Y, Hla T et al (2017). S1PR1 (sphingosine‐1‐phosphate receptor 1) signaling regulates blood flow and pressure. Hypertension 70: 426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo A, Zhang Y, Kothiya M, Galvani S, Obinata H, Bucci M et al (2015). Nogo‐B regulates endothelial sphingolipid homeostasis to control vascular function and blood pressure. Nat Med 21: 1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Lieu M, Toh BH, Kyaw TS, Bobik A, Sobey CG et al (2014). Antibodies in the pathogenesis of hypertension. Biomed Res Int 2014: 504045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Moore JP, Budzyn K, Guida E, Diep H, Vinh A et al (2012). Reversal of vascular macrophage accumulation and hypertension by a CCR2 antagonist in deoxycorticosterone/salt‐treated mice. Hypertension 60: 1207–1212. [DOI] [PubMed] [Google Scholar]
- Chan CT, Sobey CG, Lieu M, Ferens D, Kett MM, Diep H et al (2015). Obligatory role for B cells in the development of angiotensin II‐dependent hypertension. Hypertension 66: 1023–1033. [DOI] [PubMed] [Google Scholar]
- Chen J, Tang H, Sysol JR, Moreno‐Vinasco L, Shioura KM, Chen T et al (2014). The sphingosine kinase 1/sphingosine‐1‐phosphate pathway in pulmonary arterial hypertension. Am J Respir Crit Care Med 190: 1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeloth N, Bernhardt G, Hofmann F, Genth H, Forster R (2005). Sphingosine‐1‐phosphate mediates migration of mature dendritic cells. J Immunol 175: 2960–2967. [DOI] [PubMed] [Google Scholar]
- Eken A, Duhen R, Singh AK, Fry M, Buckner JH, Kita M et al (2017). S1P1 deletion differentially affects TH17 and regulatory T cells. Sci Rep 7: 12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenger M, Linneberg A, Jeppesen J (2015). Network‐based analysis of the sphingolipid metabolism in hypertension. Front Genet 6: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenger M, Linneberg A, Jorgensen T, Madsbad S, Sobye K, Eugen‐Olsen J et al (2011). Genetics of the ceramide/sphingosine‐1‐phosphate rheostat in blood pressure regulation and hypertension. BMC Genet 12: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Kihara A, Igarashi Y (2003). Distribution of sphingosine kinase activity in mouse tissues: contribution of SPHK1. Biochem Biophys Res Commun 309: 155–160. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T et al (2012). The sphingosine‐1‐phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest 122: 1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gairhe S, Joshi SR, Bastola MM, McLendon JM, Oka M, Fagan KA et al (2016). Sphingosine‐1‐phosphate is involved in the occlusive arteriopathy of pulmonary arterial hypertension. Pulm Circ 6: 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris CS, Wu L, Acharya S, Arac A, Blaho VA, Huang Y et al (2013). Defective sphingosine 1‐phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17‐mediated autoimmune neuroinflammation. Nat Immunol 14: 1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S et al (2007). Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK et al (2009). Regulation of histone acetylation in the nucleus by sphingosine‐1‐phosphate. Science 325: 1254–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CM, Mittelstadt S, Banfor P, Bousquet P, Duignan DB, Gintant G et al (2016). Sphingosine‐1‐phosphate (S1P) lyase inhibition causes increased cardiac S1P levels and bradycardia in rats. J Pharmacol Exp Ther 359: 151–158. [DOI] [PubMed] [Google Scholar]
- Hisano Y, Kobayashi N, Yamaguchi A, Nishi T (2012). Mouse SPNS2 functions as a sphingosine‐1‐phosphate transporter in vascular endothelial cells. PloS one 7: e38941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefer J, Azam MA, Kroetsch JT, Leong‐Poi H, Momen MA, Voigtlaender‐Bolz J et al (2010). Sphingosine‐1‐phosphate‐dependent activation of p38 MAPK maintains elevated peripheral resistance in heart failure through increased myogenic vasoconstriction. Circ Res 107: 923–933. [DOI] [PubMed] [Google Scholar]
- Hofmann M, Brinkmann V, Zerwes HG (2006). FTY720 preferentially depletes naive T cells from peripheral and lymphoid organs. Int Immunopharmacol 6: 1902–1910. [DOI] [PubMed] [Google Scholar]
- Itani HA, McMaster WG Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM et al (2016). Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension 68: 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang C, Koh YJ, Lim NK, Kang HJ, Kim DH, Park SK et al (2009). Angiopoietin‐2 exocytosis is stimulated by sphingosine‐1‐phosphate in human blood and lymphatic endothelial cells. Arterioscler Thromb Vasc Biol 29: 401–407. [DOI] [PubMed] [Google Scholar]
- Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J et al (2014). DC isoketal‐modified proteins activate T cells and promote hypertension. J Clin Invest 124: 4642–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Kawasaki‐Nishi S, Otsuka M, Hisano Y, Yamaguchi A, Nishi T (2018). MFSD2B is a sphingosine 1‐phosphate transporter in erythroid cells. Sci Rep 8: 4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan SM, Dowling JK, Ling YH, Diep H, Chan CT, Ferens D et al (2016). Inflammasome activity is essential for one kidney/deoxycorticosterone acetate/salt‐induced hypertension in mice. Br J Pharmacol 173: 752–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek M, Chacinska M, Chabowski A, Baranowski M (2015). Sources, metabolism, and regulation of circulating sphingosine‐1‐phosphate. J Lipid Res 56: 1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Zamora‐Pineda J, Degagne E, Saba JD (2017). S1P lyase regulation of thymic egress and oncogenic inflammatory signaling. Mediators Inflamm 2017: 7685142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis ND, Haxhinasto SA, Anderson SM, Stefanopoulos DE, Fogal SE, Adusumalli P et al (2013). Circulating monocytes are reduced by sphingosine‐1‐phosphate receptor modulators independently of S1P3 . J Immunol 190: 3533–3540. [DOI] [PubMed] [Google Scholar]
- Liao JJ, Huang MC, Goetzl EJ (2007). Cutting edge: alternative signaling of Th17 cell development by sphingosine 1‐phosphate. J Immunol 178: 5425–5428. [DOI] [PubMed] [Google Scholar]
- Lidington D, Peter BF, Meissner A, Kroetsch JT, Pitson SM, Pohl U et al (2009). The phosphorylation motif at serine 225 governs the localization and function of sphingosine kinase 1 in resistance arteries. Arterioscler Thromb Vasc Biol 29: 1916–1922. [DOI] [PubMed] [Google Scholar]
- Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA et al (2009). The receptor S1P1 overrides regulatory T cell‐mediated immune suppression through Akt‐mTOR. Nat Immunol 10: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yang K, Burns S, Shrestha S, Chi H (2010). The S1P1‐mTOR axis directs the reciprocal differentiation of TH1 and Treg cells. Nat Immunol 11: 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Milstien S, Spiegel S (2009). Sphingosine‐1‐phosphate: the Swiss army knife of sphingolipid signaling. J Lipid Res 50 (Suppl): S272–S276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ et al (2010). Interleukin 17 promotes angiotensin II‐induced hypertension and vascular dysfunction. Hypertension 55: 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Matsuyuki H, Shimano K, Kataoka H, Sugahara K, Chiba K (2007). Migration of CD4 T cells and dendritic cells toward sphingosine 1‐phosphate (S1P) is mediated by different receptor subtypes: S1P regulates the functions of murine mature dendritic cells via S1P receptor type 3. J Immunol 178: 3437–3446. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Seki N, Kataoka H, Takemoto K, Utsumi H, Fukunari A et al (2015). IL‐17‐producing Vγ4+ γδ T cells require sphingosine 1‐phosphate receptor 1 for their egress from the lymph nodes under homeostatic and inflammatory conditions. J Immunol 195: 1408–1416. [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J et al (2002). Alteration of lymphocyte trafficking by sphingosine‐1‐phosphate receptor agonists. Science 296: 346–349. [DOI] [PubMed] [Google Scholar]
- Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C et al (2010). Central and peripheral mechanisms of T‐lymphocyte activation and vascular inflammation produced by angiotensin II‐induced hypertension. Circ Res 107: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V et al (2004). Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427: 355–360. [DOI] [PubMed] [Google Scholar]
- Meissner A (2017). S1PR1 (sphingosine‐1‐phosphate receptor 1) signaling in the regulation of vascular tone and blood pressure: is S1PR1 doing the trick? Hypertension 70: 232–234. [DOI] [PubMed] [Google Scholar]
- Meissner A, Miro F, Jimenez‐Altayo F, Jurado A, Vila E, Planas AM (2017). Sphingosine‐1‐phosphate signalling – a key player in the pathogenesis of angiotensin II‐induced hypertension. Cardiovasc Res 113: 123–133. [DOI] [PubMed] [Google Scholar]
- Meissner A, Yang J, Kroetsch JT, Sauve M, Dax H, Momen A et al (2012). Tumor necrosis factor‐α‐mediated downregulation of the cystic fibrosis transmembrane conductance regulator drives pathological sphingosine‐1‐phosphate signaling in a mouse model of heart failure. Circulation 125: 2739–2750. [DOI] [PubMed] [Google Scholar]
- Mendoza A, Breart B, Ramos‐Perez WD, Pitt LA, Gobert M, Sunkara M et al (2012). The transporter Spns2 is required for secretion of lymph but not plasma sphingosine‐1‐phosphate. Cell Rep 2: 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza A, Fang V, Chen C, Serasinghe M, Verma A, Muller J et al (2017). Lymphatic endothelial S1P promotes mitochondrial function and survival in naive T cells. Nature 546: 158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian MO, Barhoumi T, Briet M, Paradis P, Schiffrin EL (2016). Deficiency of T‐regulatory cells exaggerates angiotensin II‐induced microvascular injury by enhancing immune responses. J Hypertens 34: 97–108. [DOI] [PubMed] [Google Scholar]
- Michaud J, Im DS, Hla T (2010). Inhibitory role of sphingosine 1‐phosphate receptor 2 in macrophage recruitment during inflammation. J Immunol 184: 1475–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Vinh A, Tuck KL, Sakkal S, Krishnan SM, Chan CT et al (2015). M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss, and elevated blood pressure. Am J Physiol Heart Circ Physiol 309: H906–H917. [DOI] [PubMed] [Google Scholar]
- Muller J, von Bernstorff W, Heidecke CD, Schulze T (2017). Differential S1P receptor profiles on M1‐ and M2‐polarized macrophages affect macrophage cytokine production and migration. Biomed Res Int 2017: 7584621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM (2013). Interleukin‐17 causes Rho‐kinase‐mediated endothelial dysfunction and hypertension. Cardiovasc Res 97: 696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosalski R, McGinnigle E, Siedlinski M, Guzik TJ (2017). Novel immune mechanisms in hypertension and cardiovascular risk. Curr Cardiovasc Risk Rep 11: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi S, Oda M, Matsuda H, Ikari S, Sakurai J (2004). Clostridium perfringens α‐toxin activates the sphingomyelin metabolism system in sheep erythrocytes. J Biol Chem 279: 12181–12189. [DOI] [PubMed] [Google Scholar]
- Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y et al (2007). Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine‐1‐phosphate. Science 316: 295–298. [DOI] [PubMed] [Google Scholar]
- Park SJ, Im DS (2017). Sphingosine 1‐phosphate receptor modulators and drug discovery. Biomol Ther 25: 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Angel CE, McIntosh JD, Brooks AE, Middleditch M, Chen CJ et al (2014). Sphingosine‐1‐phosphate lyase is expressed by CD68+ cells on the parenchymal side of marginal reticular cells in human lymph nodes. Eur J Immunol 44: 2425–2436. [DOI] [PubMed] [Google Scholar]
- Payne SG, Milstien S, Spiegel S (2002). Sphingosine‐1‐phosphate: dual messenger functions. FEBS Lett 531: 54–57. [DOI] [PubMed] [Google Scholar]
- Pereira JP, Xu Y, Cyster JG (2010). A role for S1P and S1P1 in immature‐B cell egress from mouse bone marrow. PloS one 5: e9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R et al (2010). Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med 207: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigogliuso S, Donati C, Cassara D, Taverna S, Salamone M, Bruni P et al (2010). An active form of sphingosine kinase‐1 is released in the extracellular medium as component of membrane vesicles shed by two human tumor cell lines. J Oncol 2010: 509329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J, Proia RL, Olivera A (2008). The alliance of sphingosine‐1‐phosphate and its receptors in immunity. Nat Rev Immunol 8: 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T (2007). Induction of vascular permeability by the sphingosine‐1‐phosphate receptor‐2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol 27: 1312–1318. [DOI] [PubMed] [Google Scholar]
- Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS et al (2004). Sphingosine 1‐phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem 279: 13839–13848. [DOI] [PubMed] [Google Scholar]
- Schulze T, Golfier S, Tabeling C, Rabel K, Graler MH, Witzenrath M et al (2011). Sphingosine‐1‐phospate receptor 4 (S1P4) deficiency profoundly affects dendritic cell function and TH17‐cell differentiation in a murine model. FASEB J 25: 4024–4036. [DOI] [PubMed] [Google Scholar]
- Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG (2005). Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309: 1735–1739. [DOI] [PubMed] [Google Scholar]
- Sensken SC, Bode C, Nagarajan M, Peest U, Pabst O, Graler MH (2010). Redistribution of sphingosine 1‐phosphate by sphingosine kinase 2 contributes to lymphopenia. J Immunol 184: 4133–4142. [DOI] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL et al (2006). CD69 acts downstream of interferon‐α/β to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440: 540–544. [DOI] [PubMed] [Google Scholar]
- Shirasuna K, Karasawa T, Usui F, Kobayashi M, Komada T, Kimura H et al (2015). NLRP3 deficiency improves angiotensin II‐induced hypertension but not fetal growth restriction during pregnancy. Endocrinology 156: 4281–4292. [DOI] [PubMed] [Google Scholar]
- Sic H, Kraus H, Madl J, Flittner KA, von Munchow AL, Pieper K et al (2014). Sphingosine‐1‐phosphate receptors control B‐cell migration through signaling components associated with primary immunodeficiencies, chronic lymphocytic leukemia, and multiple sclerosis. J Allergy Clin Immunol 134: 420–428. [DOI] [PubMed] [Google Scholar]
- Siedlinski M, Nosalski R, Szczepaniak P, Ludwig‐Galezowska AH, Mikolajczyk T, Filip M et al (2017). Vascular transcriptome profiling identifies sphingosine kinase 1 as a modulator of angiotensin II‐induced vascular dysfunction. Sci Rep 7: 44131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC (2013). Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol 14: 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino R, Bertolino A, Terlizzi M, Iacono VM, Maiolino P, Cirino G et al (2015). B cell depletion increases sphingosine‐1‐phosphate‐dependent airway inflammation in mice. Am J Respir Cell Mol Biol 52: 571–583. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S (2003). Sphingosine‐1‐phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4: 397–407. [DOI] [PubMed] [Google Scholar]
- Spijkers LJ, Alewijnse AE, Peters SL (2012). FTY720 (fingolimod) increases vascular tone and blood pressure in spontaneously hypertensive rats via inhibition of sphingosine kinase. Br J Pharmacol 166: 1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijkers LJ, van den Akker RF, Janssen BJ, Debets JJ, De Mey JG, Stroes ES et al (2011). Hypertension is associated with marked alterations in sphingolipid biology: a potential role for ceramide. PloS one 6: e21817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendeman SL, Xiong Y, Cantalupo A, Yuan H, Burg N, Hisano Y et al (2017). An engineered S1P chaperone attenuates hypertension and ischemic injury. Sci Signal 10: eaal2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabeling C, Yu H, Wang L, Ranke H, Goldenberg NM, Zabini D et al (2015). CFTR and sphingolipids mediate hypoxic pulmonary vasoconstriction. Proc Natl Acad Sci U S A 112: E1614–E1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe K, Paugh SW, Milstien S, Spiegel S (2008). “Inside‐out” signaling of sphingosine‐1‐phosphate: therapeutic targets. Pharmacol Rev 60: 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrason G, Auli M, Mustafa S, Dolgachev V, Domenech MT, Prats N et al (2011). The sphingosine‐1‐phosphate receptor‐1 antagonist, W146, causes early and short‐lasting peripheral blood lymphopenia in mice. Int Immunopharmacol 11: 1773–1779. [DOI] [PubMed] [Google Scholar]
- Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H et al (2008). Activation of sphingosine kinase‐1 reverses the increase in lung vascular permeability through sphingosine‐1‐phosphate receptor signaling in endothelial cells. Circ Res 103: 1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuy AV, Reimann CM, Hemdan NY, Graler MH (2014). Sphingosine 1‐phosphate in blood: function, metabolism, and fate. Cell Physiol Biochem 34: 158–171. [DOI] [PubMed] [Google Scholar]
- Urtz N, Gaertner F, von Bruehl ML, Chandraratne S, Rahimi F, Zhang L et al (2015). Sphingosine 1‐phosphate produced by sphingosine kinase 2 intrinsically controls platelet aggregation in vitro and in vivo. Circ Res 117: 376–387. [DOI] [PubMed] [Google Scholar]
- Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ et al (2010). Inhibition and genetic ablation of the B7/CD28 T‐cell costimulation axis prevents experimental hypertension. Circulation 122: 2529–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel P, Donoviel MS, Read R, Hansen GM, Hazlewood J, Anderson SJ et al (2009). Incomplete inhibition of sphingosine 1‐phosphate lyase modulates immune system function yet prevents early lethality and non‐lymphoid lesions. PloS one 4: e4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TM, Ishizu AN, Foo JC, Toh XR, Zhang F, Whee DM et al (2017). Mfsd2b is essential for the sphingosine‐1‐phosphate export in erythrocytes and platelets. Nature 550: 524–528. [DOI] [PubMed] [Google Scholar]
- Wacker BK, Freie AB, Perfater JL, Gidday JM (2012). Junctional protein regulation by sphingosine kinase 2 contributes to blood–brain barrier protection in hypoxic preconditioning‐induced cerebral ischemic tolerance. J Cereb Blood Flow Metab 32: 1014–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichand B, Weis N, Weigert A, Grossmann N, Levkau B, Brune B (2013). Apoptotic cells enhance sphingosine‐1‐phosphate receptor 1 dependent macrophage migration. Eur J Immunol 43: 3306–3313. [DOI] [PubMed] [Google Scholar]
- Weiler S, Braendlin N, Beerli C, Bergsdorf C, Schubart A, Srinivas H et al (2014). Orally active 7‐substituted (4‐benzylphthalazin‐1‐yl)‐2‐methylpiperazin‐1‐yl] nicotinonitriles as active‐site inhibitors of sphingosine 1‐phosphate lyase for the treatment of multiple sclerosis. J Med Chem 57: 5074–5084. [DOI] [PubMed] [Google Scholar]
- Yang J, Noyan‐Ashraf MH, Meissner A, Voigtlaender‐Bolz J, Kroetsch JT, Foltz W et al (2012). Proximal cerebral arteries develop myogenic responsiveness in heart failure via tumor necrosis factor‐α‐dependent activation of sphingosine‐1‐phosphate signaling. Circulation 126: 196–206. [DOI] [PubMed] [Google Scholar]
- Yang L, Han Z, Tian L, Mai P, Zhang Y, Wang L et al (2015). Sphingosine 1‐phosphate receptor 2 and 3 mediate bone marrow‐derived monocyte/macrophage motility in cholestatic liver injury in mice. Sci Rep 5: 13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY et al (2013). Immunosenescent CD8+ T cells and C‐X‐C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 62: 126–133. [DOI] [PubMed] [Google Scholar]
- Zamora‐Pineda J, Kumar A, Suh JH, Zhang M, Saba JD (2016). Dendritic cell sphingosine‐1‐phosphate lyase regulates thymic egress. J Exp Med 213: 2773–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YD, Chu L, Lin K, Granton E, Yin L, Peng J et al (2015). A biochemical approach to understand the pathogenesis of advanced pulmonary arterial hypertension: metabolomic profiles of arginine, sphingosine‐1‐phosphate, and heme of human lung. PloS one 10: e0134958. [DOI] [PMC free article] [PubMed] [Google Scholar]


