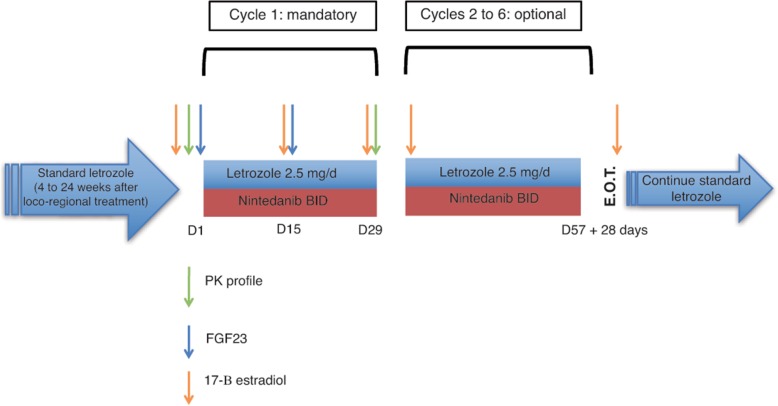
Fig. 1.
Trial design. Patients completing the treatment for early hormone receptor-positive breast cancer that were eligible for letrozole treatment and were receiving it for a minimum of 4 weeks were enrolled in the trial. This design allowed testing whether letrozole adjuvant treatment was actually achieving its therapeutic goal (17-B-estradiol suppression) and whether the concurrent administration of nintedanib exerted any negative influence on it even in the absence of pharmacokinetic interactions between both drugs. Cycle 1 was mandatory and included pharmacokinetic profiling and pharmacodynamic endpoints (FGF23 and 17-B-estradiol suppression). Cycles 2 to 6 were included in order to provide the option for any potential benefit that a long-term administration of a multi-tyrosine-kinase inhibitor could imply in the adjuvant setting, as long as patients willingly decided to continue treatment and no toxicity was observed. After completing 1 to 6 cycles, patients continued on standard adjuvant letrozole treatment. And end-of-treatment (EOT) visit was scheduled 28 days after the last nintedanib dose