Summary
Background
Immunological alterations may led to the reduction in capacity and endurance levels in elite athletes by e.g. increased susceptibility to infections. There is a need to explain the impact of intensive physical effort on the CD4+ memory T cell subsets.
Methods
Fourteen participants median aged 19 years old (range 17–21 years) were recruited form Pogoń Szczecin S.A., soccer club. They performed progressive efficiency test on mechanical treadmill until exhaustion twice: during preparatory phases to spring and autumn competition rounds. We examined the influence of exhaustive effort on the selected CD45+, especially CD4+ memory T cell subsets and inflammation markers determined before, just after the test and during recovery time.
Results
Significant changes in total CD45+ cells and decrease in T lymphocytes percentage after the run was observed. Significant fluctuations in T cells’ distribution were related not only to the changes in Th or Tc subsets but also to increase in naïve T cell percentage during recovery. Increase in TNF-α and IL-8 post-exercise, IL-6 and IL-10 plasma levels in recovery was also found.
Conclusions
The novel finding of our study is that the run performed on mechanical treadmill caused a significant release of CD4+ T naïve cells into circulation. Post-exercise increase in circulating NK cells is related with fast biological response to maximal effort. However, at the same time an alternative mechanism enhancing inflammation is involved.
Keywords: cytokines, elite soccer players, flow cytometry, lymphocytes, maximal effort
Kratak sadržaj
Uvod
Imunološke promene mogu da dovedu do umanjenja kapaciteta i nivoa otpornosti elitnih atletičara npr. usled povećane osetljivosti na infekcije. Zato je potrebno ispitati uticaj intenzivnog fizičkog napora na memoriju T ćelija CD4+.
Metode
Četrdeset učesnika fudbalskog kluba Pago Szezecin S.A. starosti srednjih godina 19 (između 17–21 godina) ipitivani su u ovom radu. Oni su izvodili progresivni test efikanosti sve do izrazitog napora dva puta: za vreme priprema u toku proleća i u toku jesenje runde. Ispitivan je uticaj takvog zamaranja na CD45+, i to specijalno na memorije T ćelije CD4+, kao i na inflamacione markere određene pre, kao i nakon testa i u periodu oporavka.
Rezultati
Uočene su značajne promene u ukupnim CD4+ ćelijama i procenta T limfocita nakon trčanja. Značajne fluktuacije u raspodeli T ćelija nisu bile samo povezane sa promenama Th ili Tc, već i povećanjem procenta T ćelija u toku oporavka. Nađeno je i povećanje nivoa u plazmi TNF-α i IL-8 nakon vežbanja, IL-6 i IL-10 nakon oporavka.
Zaključak
U ovom istraživanju nađeno je značajno povećanje CD4+ T ćelija u cirkulaciji usled mehaničkog napora. Povećanje cirkulišućih NK ćelija u post-fazi vežbanja objašnjava se brzim biološkim odgovorom na maksimalni napor. Međutim, istovremeno se uključuje i alternativni mehanizam koji dovodi do povećanja inflamacije.
Ključne reči: citokini, elitni fudbaleri, flow citometrija, limociti, maksimalni napor
Introduction
Immunological alterations may provide to the reduction in capacity and endurance levels in elite athletes. This phenomenon is associated with decreased immune function resulting in e.g. increased susceptibility to infections. It is well known that physical training requires an optimal balance between training load (intensity and volume) and recovery to improve athlete’s capacity.
During the exercise inflammatory environment arises stimulating B and T lymphocytes to proliferate and differentiate respectively to the local demand. The inflammatory state may often result in Delayed Onset Muscle Soreness (DOMS) described as pain, tenderness and stiffness of muscles with the peak of symptoms between 24 and 72 hours after the exercise (1, 2). When the inflammatory agents, as well as those cells are eliminated, memory cells remain and proliferate to ensure rapid immune response in similar circumstances in the future (3). Those memory cell populations may live for years helping to eliminate inflammatory environment and being one of the ways of adaptation to intense physical effort. Literature data point out the risk of upper respiratory system infection after exhaustive exercise (4, 5). Viral infections, for example, may lead to the state being a combination of lethargy, easy fatigability, myalgia. It is highly unfavourable in athletes and, worse, it may persist for even several months in some cases (4, 5, 6).
Such lymphocytes as natural killers (NKs), Tc (CD8+) and T cells are characterized by higher cytotoxicity and stress responsivity in comparison to Th (CD4+) or B lymphocytes. β2-adrenergic receptors and several adhesion and/or activation molecules are also more frequent in case of the first cell subsets. The presence of β2-adrenergic receptors explains the ability of binding adrenaline or noradrenaline, known stress molecules, by cytotoxic cells. After that those cells may infiltrate to the tissues under the stress. It is hypothesized that this preferential mobilization of lymphocyte with cytotoxic properties is realised to ensure immunosurveillance in stress-exposed tissues (7, 8, 9, 10).
On the other hand, chronic high-intensity exercise could stimulate T cells leading to immunosuppression (5, 6, 7, 8, 9, 10, 11). The most interesting subset of cells involved in post-effort immunological response is the CD4+ T cell pool. This seems to be related to the pleiotropic role of CD4+ cells in the induction and regulation of immune response, mainly by the synthesis and release of numerous cyto- and chemokines. These molecules act in two ways: cytokines activate adjacent cells for specific functions and chemokines recruit new immune cells (3). According to their receptors, proliferative capacity and effector function, CD4+ memory T cells have been divided into 2 populations: T central memory (TCM) and T effector memory (TEM) ones (3).
It is worth noting that, to our best knowledge, there is a lack of literature data describing the impact of intensive physical effort on the CD4+ memory T cell subsets, at least among soccer players. Some of the players asked for overall condition, especially after the soccer match, complained of DOMS symptoms, that suggested us ongoing/past inflammatory process. From this point of view, the aim of this study was to compare changes in selected CD45+ cell subsets, as well as inflammation markers after the progressive test on mechanical treadmill until exhaustion among soccer players at the beginning of the preparatory phase to spring and autumn competition round.
Centre for Human Structural and Functional Research, Faculty of Physical Education and Health Promotion, University of Szczecin, Szczecin, Poland was established to perform sport research in order to improve the routine training practice on scientific bases. We closely cooperate with a top league soccer club, namely Pogoń Szczecin S.A. From this point of view, recruiting soccer players for this experiment was a natural consequence of this cooperation.
Materials and Methods
Study design
Soccer (also referred to as football) is one of the most popular team sport discipline, watched and played by billions of people all across the world (12). From this reason, the market size of the European professional soccer, being most prominent soccer market in the world, is still growing. For example, in the 2015/16 season, the total revenue of the European professional soccer market was estimated at 24.6 billion euros (12). Moreover, the most talented and popular soccer players’ transfer fees reach millions of euros (13). Taking all these into account, there is surprisingly low data regarding analyses of biological and immunological parameters among soccer players. Therefore, we believe that the presented data would interest a number of sport physicians, physiologists and trainers, especially taking care of soccer players in professional soccer clubs.
The study was designed to better understand the influence of exhaustive effort on selected biological and immunological parameters as a comparison of two preparatory phases to competition rounds – spring and autumn ones among highly qualified elite athletes, namely soccer players.
To accomplish this experiment, complete blood count, white blood cells distribution and CD4+ memory T cell subsets (T central memory (TCM) and T effector memory (TEM)) distributions before, immediately after the progressive test, as well as during recovery time (ca. 17 hours after the test) were determined. Cardiorespiratory fitness measures: maximum oxygen uptake (VO2max), maximum heart rate (HRmax), maximum ventilation (VE), anaerobic threshold (AT), respiratory quotient (RQ; volume ratio of emitted CO2 to oxygen uptake), respiratory compensation (RC), maximal voluntary ventilation (MVV), metabolic equivalent (MET) and respiratory frequency (Rf) were also determined.
The restitution time (17 hours) was established as an average time between two consecutive training units or the soccer match and the following training unit.
The studies were duplicated and performed at the beginning of preparatory phase to spring and autumn competition rounds among soccer players in March and July 2016, respectively in the same part of the day ± 1 hour. The timing of the experiment was in line with club routine capacity tests performed. On the other hand, the players who train and play top league matches, cumulate the fatigue and the club trainers need to monitor the players to avoid the over-training syndrome. The studies were performed in the Biochemistry, as well as Physiology Laboratories, Centre for Human Structural and Functional Research, Faculty of Physical Education and Health Promotion, University of Szczecin, Szczecin, Poland.
Participants
Fourteen participants median aged 19 years old (range 17–21 years) were recruited form Pogoń Szczecin S.A., a top league soccer club in Poland. All soccer players qualified to this study were playing in a midfielder, striker or defender position during the experiment time. They had no history of any metabolic syndrome (as defined by International Diabetes Federation: diabetes, prediabetes, abdominal obesity, high cholesterol and high blood pressure) (14) or cardiovascular diseases (defined by WHO as disorders of the heart and blood vessels) (15). Participants were non-smokers and refrained from taking any medications or supplements known to affect metabolism. They (and their parents, where appropriate) were fully informed of any risks and discomfort associated with the experimental procedures before giving their consent to participate. The study was approved by the Local Ethics Committee in accordance with the Helsinki Declaration.
However, two of the participants were not able to take a part in the experiment at the beginning of the preparatory phase to spring competition round.
Participants’ body mass and body composition parameters (body mass index (BMI), basal metabolic rate (BMR), percentage of fat (FAT), fat free mass (FFM), total body water (TBW)), were determined using Body Composition Analyzer Tanita BC-418MA (Tanita, Tokyo, Japan).
The progressive test on mechanical treadmill
The progressive efficiency test on mechanical treadmill until exhaustion is one of the test routinely applied in sport practice. The advantage of treadmill run over other tests until exhaustion is the possibility of using stationary breath by breath gas exchange data analyser to determine cardiorespiratory fitness measures as a function of increasing fatigue. The test started with 5 minutes of warm-up running with the speed of 5 km/h. During the proper test the speed increased by 2 km/h after each 3 minutes of the test until exhaustion, it is until each participant refused to run because of he’s maximal fatigue. The heart rate measured at this point was noted as a HRmax. The cardiorespiratory fitness measures: maximum oxygen uptake (VO2max), maximum heart rate (HRmax), maximum ventilation (VE), anaerobic threshold (AT), respiratory quotient (RQ); respiratory compensation (RC); maximal voluntary ventilation (MVV), metabolic equivalent (MET) and respiratory frequency (Rf) were determined using state-of-the-art breath by breath gas exchange data analyser Quark CPET (Cosmed, Albano Laziale, Italy) (16). Additionally, every 3 minutes of the exercise, before increasing the workload (treadmill speed), finger capillary blood was taken for determination of lactate (LA) concentration in order to establish athlete’s anaerobic threshold. The LA concentrations were determined using mobile blood lactate monitoring system (THE EDGE Lactate Analyser, Apex Biotechnology Corp., Hsinchu, Taiwan).
Blood sampling
Blood samples were obtained tree times from the elbow vein: before the testing (pre-exercise), no longer than 5 minutes after the test (post-exercise) and ca. 17 hours after the test, at the end of recovery time (recovery). Each time, blood samples were taken into 7.5 mL S-Monovette tube with ethylenediaminetetraacetic acid (EDTA K3, 1.6 mg EDTA/mL blood) (SARSTEDT AG & Co., Nümbrecht, Germany). All analyses were performed immediately after the blood collection.
Importantly, for safety of the participants, the test protocol requires them to be after a light breakfast. Therefore, the blood samples collected after the test, with the exception for recovery time, were not fasting blood.
Haematological and biochemical analysis
Complete blood count, including number of white blood cells (WBC) and lymphocytes (LYM) was obtained using haematology analyser ABX Micros 60 (Horiba ABX, Warsaw, Poland).
Biochemical analyses were conducted with the use of an Auto Chemistry Analyser BM-100 (BioMaxima S.A., Lublin, Poland). Blood serum was used to determine total protein and creatine kinase (CK; EC 2.7.3.2) activity. The values of analysed variables were determined using a diagnostic method according to appropriate manufacturer’s protocol (BioMaxima S.A., Lublin, Poland). All analytical procedures were verified using multiparameteric control serum, as well as control serum of normal level (BioNorm) and high level (BioPath) (BioMaxima S.A., Lublin, Poland).
Flow cytometric analyses
All flow cytometric analyses were performed using BD Accuri™ C6 flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) and their results of were calculated using BD Accuri™ C6 software (ver. 1.0.264.21).
Lymphocyte subsets phenotyping in erythrocytelysed blood samples was performed using BD Multitest™ IMK kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s protocol. Briefly, two antibody cocktails (designated in our laboratory as »A« and »B«, respectively) to determine the percentages of T lymphocyte subsets (»A«) as well as lymphocyte B and NK cells (»B«) in erythrocyte-lysed blood samples were used. Cocktail »A« contained antibodies including fluorescein isothiocyanate (FITC)-labelled CD3, clone SK7; phycoerythrin (PE)-labelled CD8, clone SK1; peridinin chlorophyll protein (PerCP)-labelled CD45, clone 2D1 (HLe-1) and allophycocyanin (APC)-labelled CD4, clone SK3, whereas cocktail »B« contained FITC-labelled CD3, clone SK7; PE-labelled CD16, clone B73.1 and PE-labelled CD56, clone NCAM 16.2; PerCP-labelled CD45, clone 2D1 (HLe-1); and APC-labelled CD19, clone SJ25C1. For each sample, the fluorescence signal of at least 104 of total events was measured.
The analysis of CD4+ memory T cell subsets was performed using Human Naïve/Memory T Cell Panel (BD Pharmingen™, San Jose, CA, USA) according to manufacturer protocol. A cocktail of antibodies containing Alexa Fluor® 647-labelled Mouse Anti-Human CD197 (CCR7), clone 150503; PerCP-Cy™ 5.5-labelled Mouse Anti-Human CD4, clone SK3 and FITC-labelled Mouse Anti-Human CD45RA, clone HI100 was prepared prior to use. For each sample, the fluorescence signal of at least 2× 104 of total events was measured. Quadrants for the dot plots to determine percentages of each CD4+ memory T cell subsets were derived using appropriate fluorescence-minus-one (FMO) controls.
The measurement of selected cytokines, namely interleukin-8 (IL-8), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), tumour necrosis factor-alpha (TNF-α), and interleukin-12p70 (IL-12p70) protein levels was performed using BD Cytometric Bead Array (CBA) Human Inflammatory Cytokines Kit (BD Biosciences, San Jose, CA, USA) according to manufacturer protocol. For each sample, the fluorescence signal of 2100 events gated for capture beads population was measured. Results were calculated using FCAP Array™ Software (ver. 3.0.1; Soft Flow Hungary Ltd., Pecs, Hungary). Minimum quantifiable levels (guaranteed by the manufacturer) of cytokines that can be detected using Cytometric Bead Array are similar to the ELISA method and are equal to 3.6 pg/mL for IL-8, 7.2 pg/mL for IL-1b, 2.5 pg/mL for IL-6, 3.3 pg/mL for IL-10, 3.7 pg/mL for TNF-α, 1.9 pg/mL for IL-12p70, respectively.
Statistical analysis
All data are presented as median (interquartile range), except for the age that is presented as median (min-max). Statistical analysis was performed using STATISTICA (data analysis software system), version 12 software (StatSoft, Inc., 2014). Significance level of differences observed between spring and autumn experiment was calculated using Wilcoxon’s matched-pairs test. Significance level of differences observed between analysed time points (pre-exercise vs. post-exercise vs. recovery) was assessed using Friedman’s analysis of variance followed by post-hoc Dunn’s test with Bonferroni correction. The relationship between changes in cytokine levels and CD45+ cells’ distribution was calculated with the use of Spearman correlation coefficient analysis. Each time, P < 0.05 was considered as a significant difference.
Results
The soccer players qualified to this study were a homogenous group with similar length of training experience as well as weekly training volume. Baseline characteristic of the participants were demonstrated in Table I.
Table I.
A baseline characteristic of the participants.
| The beginning of preparatory phase to spring competition round | The beginning of preparatory phase to autumn competition round | |
|---|---|---|
| n = 12 | n = 14 | |
| Age [years] | 19 (17 – 21) | 18 (17 – 21) |
| Height [cm] | 181 (172 – 187) | 179 (172 – 184) |
| Weight [kg] | 72.4 (63.3 – 81.3) | 69.7 (63.3 – 73.1) |
| BMI [kg/m2] | 22.6 (21.6 – 23.8) | 21.5 (20.8 – 23.0) |
| BMR [kJ] | 8686 (7242 – 8782) | 7880 (7314 – 8297) |
| FAT [%] | 10.6 (6.4 – 13.1) | 9.0 (7.1 – 10.6) |
| FAT MASS [kg] | 7.90 (4.10 – 10.15) | 6.3 (4.1 – 7.9) |
| FFM [kg] | 66.2 (57.4 – 70.6) | 63.5 (58.6 – 67.1) |
| TBW [kg] | 48.5 (42.1 – 51.7) | 46.5 (42.9 – 49.1) |
| length of training experience [years] | 12 (11 – 12) | 11.5 (11 – 12) |
| weekly training volume [hours] | 10 (9 – 12) | 10.5 (10 – 12) |
The table presents median (Q1-Q3) values (except for the age, where median (min-max) is presented) characterising the participants. Body mass and body composition were determined using Body Composition Analyzer Tanita BC-418MA (Tanita, Tokyo, Japan). n – number of participants, BMI – body mass index, BMR – basal metabolic rate, FAT – percentage of fat, FFM – fat free mass, TBW – total body water.
The cardiorespiratory values found at the beginning of preparatory phase to spring and autumn round, respectively were similar to each other (Table II). There were no significant changes in VO2max, HRmax, AT, RC, MVV and MET values found in both experiments. Those results confirm the similarity of maximal effort in case of both studied time points.
Table II.
The cardiorespiratory fitness measures of participants during the progressive test until exhaustion.
| The beginning of preparatory phase to spring competition round | The beginning of preparatory phase to autumn competition round | |
|---|---|---|
| n = 12 | n = 14 | |
| VO2max [mL/kg/min] | 59.05 (56.55 – 62.03) | 60.80 (58.40 – 64.40) |
| HRmax [beats/min] | 193 (188 – 201) | 204 (190 – 210) |
| AT [beats/min] | 163 (156 – 169) | 166 (161 – 181) |
| RQ | 1.03 (1.02 – 1.06) | 1.08 (1.07 – 1.09)* |
| RC | 176 (169 – 183) | 187 (173 – 193) |
| VE [L/min] | 108.5 (99.1 – 127.3) | 150.4 (122.2 – 160.9)* |
| MVV [L/min] | 189.5 (176.2 – 194.4) | 188.2 (172.5 – 201.1) |
| MET [mL/kg/min] | 16.5 (16.1 – 16.9) | 17.3 (16.7 – 18.4) |
| Rf | 49.7 (42.4 – 52.4) | 61.5 (56.1 – 67.5)* |
The table presents median (Q1-Q3) values. The analyses were performed using state-of-the-art breath by breath gas exchange data analyser Quark CPET (Cosmed, Albano Laziale, Italy).
n – number of participants, VO2max – maximum oxygen uptake; HRmax – maximum heart rate; AT – anaerobic threshold; RQ – respiratory quotient (volume ratio of emitted CO2 to oxygen uptake); RC – respiratory compensation; VE – minute ventilation; MVV – maximal voluntary ventilation; MET – metabolic equivalent; Rf – respiratory frequency. Significance level of differences observed between spring and autumn experiment was calculated using Wilcoxon’s matched-pairs test. *P < 0.05.
There were significant changes in total white blood cells and number of lymphocytes in both experiments, yet the most probable explanation is the after-exercise dehydration of the participants, as confirmed by similar changes in serum total protein concentration (Table III).
Table III.
Selected haematological and biochemical parameters of participants during the progressive test until exhaustion.
| The beginning of preparatory phase to spring competition round | The beginning of preparatory phase to autumn competition round | |||||
|---|---|---|---|---|---|---|
| n = 12 | n = 14 | |||||
| Pre-exercise | Post-exercise | Recovery | Pre-exercise | Post-exercise | Recovery | |
| WBC [109/L] | 5.5 a)*** (5.1 – 6.0) | 7.8 b)* (6.2 – 10.0) | 6.2 (5.6 – 6.8) | 6.4 a)** (5.8 – 7.0) | 9.0 b)**** (7.7 – 11.3) | 5.8 (5.1 – 6.1) |
| LYM [109/L] | 1.6 a)* (1.1 – 1.9) | 2.7 (1.9 – 3.4) | 1.6 (1.4 – 2.1) | 1.9 a)** (1.8 – 2.2) | 3.3 b)*** (2.9 – 4.0) | 1.8 (1.7 – 2.1) |
| Total protein [g/L] | 69.43 a)*** (67.64 – 71.92) | 71.06 b)* (67.73 – 76.16) | 67.29 (64.42 – 69.13) | 71.69 a)** (69.34 – 72.29) | 75.26 b)***** (73.70 – 75.78) | 69.30 (67.50 – 71.01) |
| CK [U/L] | 262.0 a)* (239.1 – 440.7) | 354.1 (285.2 – 504.7) | 484.2 c)** (368.6 – 564.5) | 185.7 a)** (116.0 – 248.1) | 226.4 (139.1 – 370.3) | 322.1 c)*** (276.4 – 411.2) |
The table presents median (interquartile range) values. The analyses were performed using haematology analyser ABX Micros 60 (Horiba ABX, Warsaw, Poland) or Auto Chemistry Analyser BM-100 (BioMaxima S.A., Lublin, Poland), respectively.
Significance level of differences observed between analysed time points (pre-exercise vs. post-exercise vs. recovery) was assessed using Friedman’s analysis of variance followed by post-hoc Dunn’s test with Bonferroni correction.
WBC, number of white blood cells; LYM, number of lymphocytes; CK, creatine kinase activity.
The results of post-hoc analyses: a) pre-exercise vs. post-exercise, b) post-exercise vs. recovery, c) pre-exercise vs. recovery * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001; ***** P < 0.00001 n – number of participants.
Significant increases in creatine kinase (CK; EC 2.7.3.2) activities after the exercise but also in recovery were observed at the beginning of preparatory phase to spring and autumn competition round (Table III).
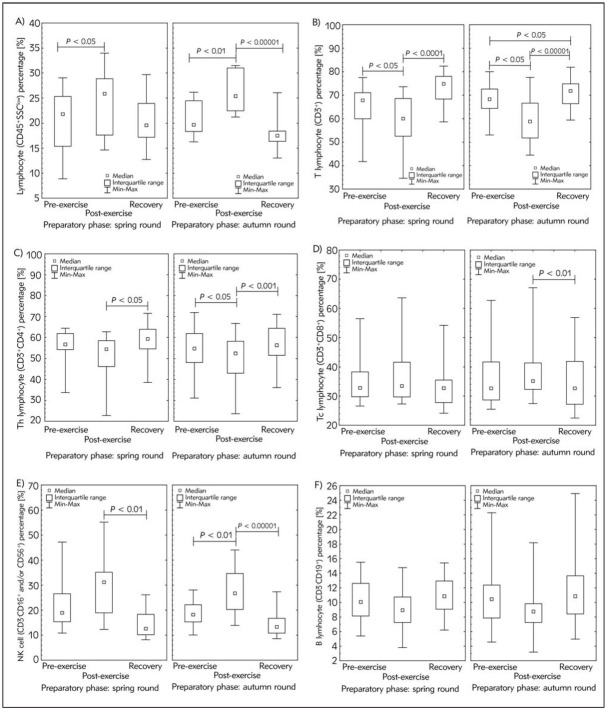
A significant increase in total CD45+ cell percentage after maximal effort was observed in peripheral blood of studied participants at the beginning of preparatory phase to spring (P < 0.05) as well as autumn (P < 0.01) competition round (Figure 1A). The decrease of these cells in recovery time (as compared to post-exercise values) was significant only in autumn (P < 0.00001). Also, significant (P < 0.05) decrease in percentage of T (CD3+) cells after the exercise test was observed in both analysed time points (spring and autumn) (Figure 1B). The values found in recovery time were significantly higher than their post-effort counterparts (P < 0.0001 and P < 0.00001 for spring and autumn, respectively). Changes observed in Th (CD3+CD4+) cells’ distribution involved the post-exercise decrease (significant only in autumn experiment; P < 0.05) and then increase in recovery time (Figure 1C). Surprisingly, no significant changes in Tc (CD3+CD8+) cells’ distribution (except for decrease in those cell subset in recovery time as compared to post-exercise in autumn) during the experiment were found (Figure 1D). It explains the changes in CD4/CD8 ratio observed after the exercise (1.6 and 1.5 for spring and autumn experiment, respectively) in comparison to baseline values (1.7 for both time points). It seems that the changes in CD45+ cells’ distribution predominantly manifest the alterations in NK (CD16+ and/or CD56+) cells (Figure 1E), since the pattern of changes was very similar in both cell subsets. The median values of NK cells’ percentage just after the exercise were ca. 1.7-fold and 1.5-fold higher than before the test (for the beginning of preparatory phase to spring and autumn competition round, respectively) and were decreasing in the recovery time (Figure 1E). No significant changes in CD19+ cells (B lymphocytes) were observed in case of all studied time points (Figure 1F).
Figure 1.
Median of the percentage of white blood cells population: A) lymphocyte (CD45+) subsets, including: B) T cells (CD3+), C) helper/inducer T cells (Th; CD3+CD4+), D) suppressor/cytotoxic T cells (Tc; CD3+CD8+), E) natural killer cells (NK; CD3–CD16+ and/or CD56+) and F) B cells (CD19+) of studied participants’ blood samples during the experiment at the beginning of preparatory phase to spring and autumn competition round, respectively.
Blood immunophenotyping protocol was performed using commercial antibodies assay kit (BD Multitest IMK Kit) according to the manufacturer instructions and analysed using BD Accuri™ C6 flow cytometer.
The midpoint represents median; box represents interquartile range; whiskers represent min-max range. Significance level of differences observed between spring and autumn experiment was calculated using Wilcoxon’s matched-pairs test. Significance level of differences observed between analysed time points (pre-exercise vs. post-exercise vs. recovery) was assessed using Friedman’s analysis of variance followed by post-hoc Dunn’s test with Bonferroni correction.
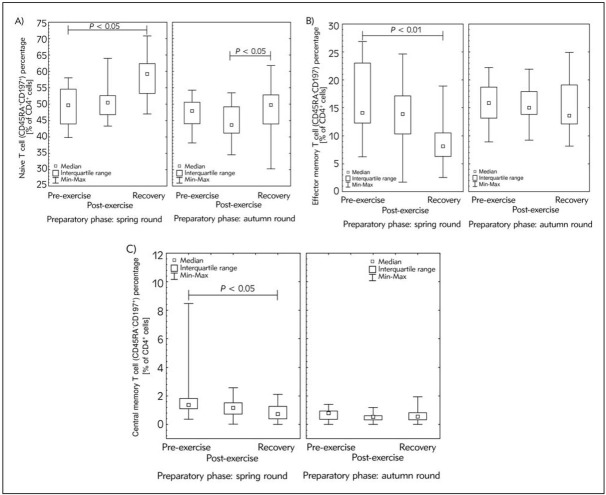
Our study demonstrated that significant fluctuations in T cells’ distribution were related not only to the changes in Th or Tc subsets but also to significant increasing in T naïve cell (CD4+CD45RA+CD197+) percentage during the recovery time (Figure 2A). Interestingly, the percentage of T naïve cells just after the exercise test, as well as in recovery time were higher in the first experiment, namely at the preparatory phase to spring competition round than the second one (conducted at the preparatory phase to the autumn competition round). Moreover, the T memory cells’ distribution ratios (TCM to TEM values for pre-exercise, post-exercise and recovery, respectively) found during preparatory phase to spring round were ca. 2-fold higher than during preparatory phase to autumn round (Figure 2B, 2C). This observation may be a valuable premise that the decrease in TCM/TEM ratio may be a hallmark of athletes’ immune system adaptation to the exercise.
Figure 2.
Median of the percentage of CD4+ memory T cell subsets: A) T Naïve cells (CD45RA+CD197+), B) T effector memory cells (TEM; CD45RA-CD197-) and C) T central memory cells (TCM; CD45RA-CD197+) of studied participants’ blood samples during the experiment at the beginning of preparatory phase to spring and autumn competition round, respectively. CD4+ memory T cell subsets analysis protocol was performed using commercial antibodies assay kit (Human Naïve/Memory T Cell Panel) according to the manufacturer instructions and analysed using BD Accuri™ C6 flow cytometer. The midpoint represents median; box represents inter quartile range; whiskers represent min-max range. Significance level of differences observed between spring and autumn experiment was calculated using Wilcoxon’s matched-pairs test. Significance level of differences observed between analysed time points (pre-exercise vs. post-exercise vs. recovery) was assessed using Friedman’s analysis of variance followed by post-hoc Dunn’s test with Bonferroni correction.
To better understand the mechanism of immunological response to the maximal effort, plasma levels of selected human cytokines were determined (Table IV), yet the IL-1 plasma levels were below method’s detection limit in all analysed time points. A significant increase in TNF-α and IL-8 plasma level just after the exercise (post-exercise) test was found at the beginning of the preparatory phase to both spring and autumn competition rounds. TNF-α levels were ca. 1.6-fold higher than baseline values (pre-exercise) and IL-8 levels were nearly 2-fold higher than the pre-exercise values. Moreover, a significant increase in IL-6 and IL-10 plasma levels in recovery time (recovery) as compared to pre-exercise as well as post-exercise values, was also found. The IL-6 and IL-10 levels observed in recovery time were almost twice as high as the pre-exercise and post-exercise ones in case of both experiments (preparatory phase to spring and autumn round). On the other hand, functional form of IL-12, namely IL-12p70 post-exercise and recovery plasma concentration was lower than its pre-exercise value in both analysed preparatory phases. Correlation analysis revealed strong correlation between IL-12p70 and CD4+ T naïve (R = -0.70, P = 0.0114) and both memory cell subsets (R = 0.68, P = 0.0158 and R = 0.60, P = 0.0386 for TCM and TEM cells, respectively) but only post-exercise in spring experiment. There were also correlations between IL-6 and CD4+ TCM (R = 0.73, P = 0.0068) and CD4+ TEM (R = 0.61, P = 0.0358) cells as well as between IL-8 and NK cells (R = 0.68, P = 0.0153) in this time-point of the experiment. At the beginning of the preparatory phase to autumn competition round, only the correlations between IL-12p70 and T lymphocytes (R = 0.61, P = 0.0195) as well as TNF and NK cells (R = 0.58, P = 0.0284) post-exercise were observed.
Table IV.
The plasma cytokine profile of participants during the progressive test until exhaustion.
| The beginning of preparatory phase to spring competition round | The beginning of preparatory phase to autumn competition round | |||||
|---|---|---|---|---|---|---|
| n = 12 | n = 14 | |||||
| Pre-exercise | Post-exercise | Recovery | Pre-exercise | Post-exercise | Recovery | |
| TNF-α [pg/mL] | 1.79 a)** (1.39 – 2.20) | 2.98 (2.60 – 3.33) | 2.72 c)* (2.22 – 3.06) | 1.90 a)* (1.68 – 2.05) | 2.94 b)**** (2.68 – 3.08) | 1.53 (1.34 – 1.57) |
| IL-8 [pg/mL] | 14.47 a)* (13.47 – 15.53) | 26.02 b)*** (23.47 – 28.14) | 12.62 (11.49 – 13.54) | 13.62 a)** (13.15 – 14.60 | 25.28 b)**** (23.76 – 26.38) | 12.34 (11.97 – 13.16) |
| IL-6 [pg/mL] | 12.26 (11.68 – 13.68) | 12.30 b)* (11.75 – 13.19) | 20.22 c)** (20.00 – 21.56) | 11.91 (11.1 – 11.79 | 11.37 b)*** (11.03 – 11.76) | 22.19 c)*** (21.64 – 22.64) |
| 2.84 (2.78 – 3.30) | 2.99 b)** (2.26 – 3.06) | 5.57 c)** (5.29 – 6.09) | 0.83 (1.78 – 0.57) | 1.02 b)** (0.77 – 1.25) | 2.15 c)*** (1.99 – 2.28) | |
| IL-12p70 [pg/mL] | 2.12 (1.98 – 2.20) | 1.66 (1.20 – 2.06) | 1.09 c)** (0.98 – 1.21) | 2.00 a)**** (1.88 – 2.21) | 0.54 b)* (0.51 – 0.78) | 1.10 c)* (0.97 – 1.28) |
The table presents median (Q1-Q3) values.
The analyses were performed using BD Cytometric Bead Array (CBA) Human Inflammatory Cytokines Kit and BD Accuri™ C6 flow cytometer.
Significance level of differences observed between analysed time points (pre-exercise vs. post-exercise vs. recovery) was assessed using Friedman’s two-way analysis of variance followed by post-hoc Dunn’s test with Bonferroni correction.
The results of post-hoc analyses:
a) pre-exercise vs. post-exercise
b) post-exercise vs. recovery
c) pre-exercise vs. recovery
* P < 0.05; ** P < 0.01; ***P < 0.001; **** P < 0.0001
n – number of participants.
Discussion
Although, the idea of immune modulation in response to exercise is not novel, there is still a lack of data confirming the mechanisms of these changes. This is particularly true concerning the adaptive area of immune system and immunosuppression effect of high-intensity exercise alone, as well as combined with long-term effort. Our previous research showed that high-intensity exercise of specialised (trained) movement did not cause an inflammation among highly qualified athletes (data submitted for publication). Significant changes in white blood cells’ distribution during the competition round were also observed. On the other hand, it is well known that muscle damage is a consequence of intense exercise. Appearing soreness and swelling of the muscle tissue, as well as release of e.g. creatine kinase (CK; EC 2.7.3.2) out of the muscle cells are the hallmarks of ongoing inflammation (17, 18, 19, 20). Our data (increases in serum CK activities) confirm the inflammation state in analysed participants. One of the features of immune system aging are changes in T cell subpopulations, namely memory, effector-memory and senescent T cells (21).
Post-effort lymphocytopenia (decrease in blood lymphocyte count below the values found before the exercise) occurring usually 0.5-1 hour after the exercise is a known fact (10, 22, 23, 24, 25, 26). The decrease in lymphocytes depends on intensity of the exercise and after maximal effort the values may drop below clinically lower limits (22). Lymphocyte count typically reach their starting values up to 1 day after the effort. However, the mechanisms of this phenomenon are not fully understood (9). The post-exercise increase in total lymphocytes was observed in both experiments during our study. However, the same phenomenon was observed in regards of white blood cell count and serum total protein concentration indicating that it was due to the post-exercise dehydration.
The main aim of the present study was to compare changes in selected CD45+ cell subsets as well as inflammation markers. The novel finding of our study is that the run being a highly important part of soccer game and performed by soccer players on a mechanical treadmill caused a significant release of CD4+ T naïve cells to circulation. Moreover, the changes in CD3+ cells’ distribution indicated a modulating effect of the progressive test used in our experiments. Navalta et al. found that three days of repeated intense interval exercise to exhaustion performed by sedentary subjects caused different changes, regarding apoptosis and migration in CD4+, CD8+, and CD19+ lymphocytes (27). They observed that CD4+ lymphocytes were the most unaffected cell subset, since the changes occurred only after the last day of exercise protocol (27), which is in opposite to our findings. However, our participants were athletes from a top league soccer club. From this point of view, the changes found in our study suggest that adaptive mechanism of immune system is related to regulatory effect of T cells, especially the CD4+ ones. Brown et al. (28) found that the redistribution of senescent CD4+ and CD8+ T lymphocytes was lower in the trained soccer players compared with untrained group after maximal exercise. There is data indicating that there were more naïve CD8+ and less senescent CD4+ and CD8+ T cells among resting athletes with high VO2max (maximal aerobic capacity) values in comparison to their counterparts with lower VO2max values (21). Simpson (9) suggested, that immune system in regular training subjects creates space for naïve T lymphocytes by mobilisation of senescent T cells. He explained this phenomenon by a feedback loop: the decrease in peripheral T lymphocytes is caused by apoptosis of senescent T cells; this gap is filled up by naïve T lymphocytes leaving thymus (9). It was assumed by Brown et al. (28) that such feedback loop should be more intense in regularly training participants. He also confirmed this assumption observing dull senescent T lymphocyte response and concomitant increase in naïve T cells among trained individuals compared to untrained counterparts (28). Our results also support this theory. We have observed that increasing time of training experience (the tests were performed during two consecutive preparatory phases) resulted in decreasing in the memory CD4+ T cells’ distribution (TCM/TEM ratio) which confirms the contribution of T cells in immune response as one of adaptation mechanisms to repeated training loads. Moreover, Bigley et al. (29) showed association between naïve CD8+ T cells and VO2max values indicating that aerobic fitness influence the proportions of these cells. They also prove that aerobic fitness have greater impact on affecting the T cell phenotypic alterations than aging (29).
The apparent change observed in NK cells’ distribution after the exercise might result from cellular migration into damaged muscle tissue and induction of pro-inflammatory cytokines release, especially that the increased serum CK activities were observed. Similar data was provided by Bigley et al. (29) who observed a stepwise redistribution of NK cells during the exercise. According to them, the explanation of this phenomenon is a possible mechanism of immunosurveillance enhancing required throughout the after-exercise recovery (29).
To better understand the effect of maximal effort on organism’s immune response including observed changes in studied CD45+ cells’ distribution, plasma levels of selected human cytokines were determined. The panel used in our analyses included pro-inflammatory cytokines, namely IL-6, IL-12p70 (3, 30, 31), anti-inflammatory IL-10 (31, 32), multifunctional cytokines: tumour necrosis factor alpha (TNF-α) and interleukin 1β (IL-1β) (3), as well as a chemokine IL-8 (32). The all play a crucial role in activation of studied cells and also are related to haematopoiesis stimulation.
TNF-α and IL-1β are known to be involved in inflammation response to muscle damage (30). There were no detectable levels of IL-1β in plasma of high qualified soccer players in our experiment but significant increase in TNF-α concentration was observed during the study. It must be emphasised that the concentration of IL-6 found in our study was more than 5-fold higher that TNF-α level. There are literature data suggesting that IL-6 inhibits the production of TNF-α and IL-1 (3, 30, 32).
It is also known that IL-10 emerges in the circulation after the exercise. It participates in anti-inflammatory response to the exercise (31, 32) playing crucial role in muscle tissue regeneration (33). Many authors reported this phenomenon after intense and prolonged exercise (33, 34, 35, 36). It is in line with our observations during both analysed preparatory phases. It is explained that increased IL-10 plasma concentration after the exercise is required for the reduction of muscle damage as well as the tissue recovery (30, 34). There are data showing relationship between the decrease in TNF-α, IL-1β, IL-6 and IL-8 serum levels and IL-10 concentration (37, 38).
The post-exercise increase in IL-10 plasma concentration could also explain a significant decrease in IL-8 level during recovery time observed in our experiments. Knowing that IL-8 and IL-6 are cytokines responsible for stimulation of haematopoiesis, the increased post-exercise IL-8 concertation and rising IL-6 concentration during the recovery time may be functional explanation of releasing CD4+ naïve T cells into circulation. On the other hand, ca. 2-fold higher IL-6 level observed during recovery time seems to confirm the hypothesis that IL-6 released from contracting muscle during the exercise acts in a hormone-like manner and mobilizes extracellular substrates and/or augments substrate delivery (32). It seems that pro-inflammatory effect of IL-6 is related more with the anabolic effect of maximal effort than with inflammation. This speculation is confirmed by post-exercise and recovery IL-12p70 plasma concentrations, which were lower than their baseline (pre-exercise) values. This was observed in both (spring and autumn) experiments.
Our data showed that there is a biological balance between pro- and anti-inflammatory effects of acute effort in highly qualified soccer players regardless the time of preparatory phase (spring or autumn) to competition round. The observed cytokine levels might help to explain the changes in CD45+ cells’ distribution after the maximal effort (until exhaustion), inducing alterations in CD4+ T cells’ distribution, especially the increase in CD4+ T naïve cells release. However, the correlation analysis results differ between spring and autumn experiment. There fore, further, broader research is needed to understand the athletes organisms’ response to the maximal effort. It also seems that post-exercise increase in circulating NK cells is related with fast biological response to maximal effort. However, at the same time there is an alternative mechanism involved and its role is to enhance the inflammation. Apparently, the second mechanism is related to the transduction of cell signalling by CD4+ cells and more extended research in this field is needed to fully understand this phenomenon.
Regarding the limitation of the study, we did not determine past cytomegalovirus, Epstein-Barr virus or herpes simplex virus-1 infections. However, we would like to emphasise that we did compare the same athletes in two experiment time-points, namely spring and autumn. We can assume that even if any of above mentioned viral infections interfered the results, it took place in both experiments. For the same reason we did not compared these results to control group. The diet was not monitored. However, the club routine includes meals (breakfast and dinners), so all the participants eat the same products. We also did not control sleep time. The soccer players were asked to behave »typically« before and during the experiments. Our study took place during regular season routine of the soccer club and therefore we could not alter this routine form one hand and did not want to, since we would like to analyse athletes during their typical training macrocycle from the other.
Perspectives
The proposal of the study has been widely discussed with the trainers working in the club. They were interested in broadening the knowledge about cellular response to exhaustive effort. They agreed that these types of studies may help verify »The Open Window Theory« and avoid of upper respiratory tract infections excluding soccer players from the games and weakening the whole team. They also confirmed that the players more often get infectious during the spring than autumn competition round. We believe that our research could broaden the knowledge about organisms’ response to exhaustive effort. But, more importantly, it could help to individualize and adapt the training process to achieve even better results, which is the main aim of combining sport and science.
Acknowledgments
The authors would like to thank the individuals who volunteered to participate in the study. We also thank the Pogoń Szczecin S.A. authorities for kind cooperation during the study.
List of abbreviations
- AT
anaerobic threshold
- BMI
body mass index
- BMR
basal metabolic rate
- CK
creatinine kinase
- DOMS
delayed onset muscle soreness
- FAT
percentage of fat
- FFM
fat free mass
- HRmax
maximum heart rate
- MET
metabolic equivalent
- MVV
maximal voluntary ventilation
- RC
respiratory compensation
- Rf
respiratory frequency
- RQ
respiratory quotient
- TBW
total body water
- VE
maximum ventilation
- VO2max
maximum oxygen uptake
Footnotes
Conflict of interest
Conflict of interest statement: The authors stated that they have no conflicts of interest.
References
- 1.Kruszyniewicz J, Skonieczna-Żydecka K, Sroka R, Adler G. The Analgesic Efficacy of Kinesiology Taping in Delayed Onset Muscle Soreness (DOMS) Centr Eur J Sport Sci Med. 2016;13:73–9. [Google Scholar]
- 2.Mizumura K, Taguchi T. Delayed onset muscle soreness: Involvement of neurotrophic factors. J Physiol Sci. 2016;66:43–52. doi: 10.1007/s12576-015-0397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pennock ND, White JT, Cross EW, Cheney EE, Tamburini BA, Kedl RM. T cell responses: naïve to memory and everything in between. Adv Physiol Educ. 2013;37:273–83. doi: 10.1152/advan.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kostrzewa-Nowak D, Nowak R, Chamera T, Buryta R, Moska W, Cięszczyk P. Post-effort chances in C-reactive protein level among soccer players at the end of the training season. J Strength Cond Res. 2015;29:1399–405. doi: 10.1519/JSC.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 5.Collinson P. Laboratory medicine is faced with the evolution of medical practice. J Med Biochem. 2017;36:211–15. doi: 10.1515/jomb-2017-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maffulli N, Testa V, Capasso G. Post-viral fatigue syndrome. A longitudinal assessment in varsity athletes. J Sports Med Phys Fitness. 1993;33:392–9. [PubMed] [Google Scholar]
- 7.Anane LH, Edwards KM, Burns VE, Drayson MT, Riddell NE, van Zanten JJ. Mobilization of gammadelta T lymphocytes in response to psychological stress, exercise, and betaagonist infusion. Brain Behav Immun. 2009;23:823–9. doi: 10.1016/j.bbi.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Atanackovic D, Schnee B, Schuch G, Faltz C, Schulze J, Weber CS. Acute psychological stress alerts the adaptive immune response: stress-induced mobilization of effector T cells. J Neuroimmunol. 2006;176:141–52. doi: 10.1016/j.jneuroim.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Simpson RJ. Aging, persistent viral infections, and immunosenescence: can exercise »make space«. Exerc Sport Sci Rev. 2011;39:23–33. doi: 10.1097/JES.0b013e318201f39d. [DOI] [PubMed] [Google Scholar]
- 10.Turner JE, Aldred S, Witard O, Drayson MT, Moss PM, Bosch JA. Latent cytomegalovirus infection amplifies CD8 T-lymphocyte mobilisation and egress in response to exercise. Brain Behav Immun. 2010;24:1362–70. doi: 10.1016/j.bbi.2010.07.239. [DOI] [PubMed] [Google Scholar]
- 11.Ziemann E, Zembron-Lacny A, Kasperska A, Antosiewicz J, Grzywacz T, Garsztka T. Exercise training-induced changes in inflammatory mediators and heat shock proteins in young tennis players. J Sports Sci Med. 2013;12:282–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Soccer – Statistics & Facts. 2018. www.statista.com/topics/1595/soccer/ Available via statista. Accessed 04 Apr.
- 13.Soccer player transfers by highest all-time transfer fee worldwide as of February. 2018. https://www.statista.com/statistics/263304/transfer-fees-the-10-most-expensive-transfers-in-soccer-ever/ (in million euros). Available via statista. Accessed 04 Apr 2018.
- 14.The IDF. consensus worldwide definition of the metabolic syndrome. 2014. http://www.idf.org/metabolic-syndrome Available via IDF. Accessed 24 July.
- 15.WHO. Cardiovascular diseases. 2014. http://www.who.int/topics/cardiovascular_diseases/en/Accessed Available via WHO. 24 July.
- 16.Beaver WL, Wassermann K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1985;60:2020–7. doi: 10.1152/jappl.1986.60.6.2020. 1986; [DOI] [PubMed] [Google Scholar]
- 17.Nosaka K, Lavender A, Newton M, Sacco P. Muscle damage in resistance training – Is Muscle Damage Necessary for Strength Gain and Muscle Hypertrophy. Int J Sport Health Sci. 2003;1:1–8. [Google Scholar]
- 18.Sayers SP, Clarkson PM. Short-term immobilization after eccentric exercise. Part II: creatine kinase and myoglobin. Med Sci Sports Exerc. 2003;35:762–8. doi: 10.1249/01.MSS.0000064933.43824.ED. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki K, Totsuka M, Nakaji S, Yamada M, Kudoh S, Liu Q. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J Appl Physiol. 1985;87:1360–7. doi: 10.1152/jappl.1999.87.4.1360. 1999; [DOI] [PubMed] [Google Scholar]
- 20.Suzuki K, Yamada M, Kurakake S, Okamura N, Yamaya K, Liu Q. Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. Eur J Appl Physiol. 2000;81:281–7. doi: 10.1007/s004210050044. [DOI] [PubMed] [Google Scholar]
- 21.Spielmann G, McFarlin BK, O'Connor DP, Smith PJ, Pircher H, Simpson RJ. Aerobic fitness is associated with lower proportions of senescent blood T-cells in man. Brain Behav Immun. 2011;25:1521–9. doi: 10.1016/j.bbi.2011.07.226. [DOI] [PubMed] [Google Scholar]
- 22.Simpson RJ, Florida-James GD, Whyte GP, Guy K. The effects of intensive, moderate and downhill treadmill running on human blood lymphocytes expressing the adhesion/activation molecules CD54 (ICAM-1), CD18 (beta2 integrin) and CD53. Eur J Appl Physiol. 2006;97:109–21. doi: 10.1007/s00421-006-0146-4. [DOI] [PubMed] [Google Scholar]
- 23.Simpson RJ, Florida-James GD, Cosgrove C, Whyte GP, Macrae S, Pircher H. High-intensity exercise elicits the mobilization of senescent T lymphocytes into the peripheral blood compartment in human subjects. J Appl Physiol. 1985;103:396–401. doi: 10.1152/japplphysiol.00007.2007. 2007; [DOI] [PubMed] [Google Scholar]
- 24.Simpson RJ, Florida-James GD, Whyte GP, Black JR, Ross JA, Guy K. Apoptosis does not contribute to the blood lymphocytopenia observed after intensive and downhill treadmill running in humans. Res Sports Med. 2007;15:157–74. doi: 10.1080/15438620701405339. [DOI] [PubMed] [Google Scholar]
- 25.Simpson RJ, Cosgrove C, Ingram LA, Florida-James GD, Whyte GP, Pircher H. Senescent T-lymphocytes are mobilised into the peripheral blood compartment in young and older humans after exhaustive exercise. Brain Behav Immun. 2008;22:544–51. doi: 10.1016/j.bbi.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Simpson RJ, Cosgrove C, Chee MM, McFarlin BK, Bartlett DB, Spielmann G. Senescent phenotypes and telomere lengths of peripheral blood T-cells mobilized by acute exercise in humans. Exerc Immunol Rev. 2010;16:40–55. [PubMed] [Google Scholar]
- 27.Navalta JW, Tibana RA, Fedor EA, Vieira A, Prestes J. Three consecutive days of interval runs to exhaustion affects lymphocyte subset apoptosis and migration. Bio med Res Int. 2014;2014:694801. doi: 10.1155/2014/694801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown FF, Bigley AB, Sherry C, Neal CM, Witard OC, Simpson RJ. Training status and sex influence on senescent T-lymphocyte redistribution in response to acute maximal exercise. Brain Behav Immun. 2014;39:152–9. doi: 10.1016/j.bbi.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 29.Bigley AB, Rezvani K, Chew C, Sekine T, Pistillo M, Crucian B. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav Immun. 2014;39:160–71. doi: 10.1016/j.bbi.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 30.Hirose L, Nosaka K, Newton M, Laveder A, Kano M, Peake J. Changes in inflammatory mediators following eccentric exercise of the elbow flexors. Exerc Immunol Rev. 2004;1:75–90. [PubMed] [Google Scholar]
- 31.Ghafourian M, Ashtary-Larky D, Chinipardaz R, Eskandary N, Mehavaran M. () Inflammatory Biomarkers' Response to Two Different Intensities of a Single Bout Exercise Among Soccer Players. Iran Red Crescent Med J. 2016;18:e21498. doi: 10.5812/ircmj.21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 1985;98:1154–62. doi: 10.1152/japplphysiol.00164.2004. 2005; [DOI] [PubMed] [Google Scholar]
- 33.Malm C. Exercise immunology: a skeletal muscle perspective. Exerc Immunol Rev. 2002;8:116–67. [PubMed] [Google Scholar]
- 34.Fatouros IG, Jamurtas AZ. Insights into the molecular etiology of exercise-induced inflammation: opportunities for optimizing performance. J Inflamm Res. 2016;9:175–86. doi: 10.2147/JIR.S114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki K, Nakaji S, Yamada M, Liu Q, Kurakake S, Okamura N. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med Sci Sports Exerc. 2003;35:348–55. doi: 10.1249/01.MSS.0000048861.57899.04. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki K, Nakaji S, Yamada M, Totsuka M, Sato K, Sugawara K. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev. 2002;8:6–48. [PubMed] [Google Scholar]
- 37.Mezil YA, Allison D, Kish K, Ditor D, Ward WE, Tsiani E. Response of Bone Turnover Markers and Cytokines to High-Intensity Low-Impact Exercise. Med Sci Sports Exerc. 2015;47:1495–502. doi: 10.1249/MSS.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 38.Pajkrt D, Manten A, van der Poll T, Tiel-van Buul MM, Jansen J, Wouter ten Cate J. Modulation of cytokine release and neutrophil function by granulocyte colonystimulating factor during endotoxemia in humans. Blood. 1997;90:1415–24. [PubMed] [Google Scholar]