Fig. 4.
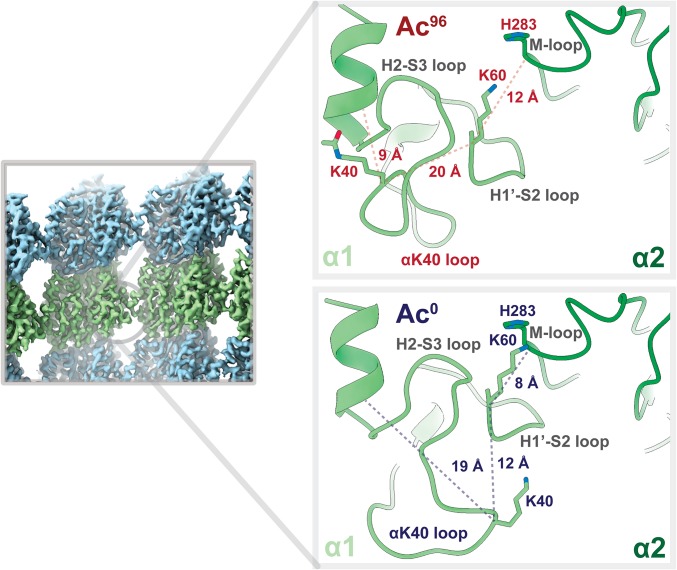
Acetylation may weaken lateral interactions. Close-up view of the lateral contacts between two α-tubulin monomers at a nonseam location (α1, light green; α2, dark green). K40 in α1 of the Ac0 state is 8 Å closer to the M-loop of α2 and appears to buttress the H1′–S2 loop, providing support for the vital α1K60–α2H283 lateral interaction. By contrast, that support is lost in the Ac96 state because the acetylated K40 now packs much closer to the hydrophobic, inner core.