Abstract
Purpose of Review
Hepatitis C virus (HCV) infection has been the leading cause of cirrhosis in the US now for the last several decades. With the introduction of highly effective direct acting antiviral (DAA) drugs, cure rates are now almost 100%. With this explosion of effective therapy, it is possible that many patients with HCV may have reversion in fibrosis. The purpose of this review is therefore to report on recent findings in this field.
Recent Findings
Older data that examined the effect of interferon based HCV therapy indicate that fibrosis reverses after HCV eradication. More recent work in the DAA era similarly indicates that fibrosis is reversible. A caveat is that DAA therapy causes rapid viral clearance, and appears to lead to rapid reductions in inflammation. Some tools (such as transient elastography) which may also reflect the inflammatory response, and thus may “overestimate” of fibrosis reversal. However, emerging data suggesting improved outcomes in patients with cirrhosis after HCV clearance support the concept that even cirrhosis reverses in some patients.
Summary
Fibrosis (and cirrhosis) reversion, to some extent, occurs after HCV clearance. This topic is vitally important and information continues to emerge; more data on this subject are expected and needed.
Keywords: cirrhosis, reversion, stellate cell, extracellular, matrix
Introduction
Chronic injury to the liver ultimately leads to fibrosis and cirrhosis and can result from any of several different diseases, including viral hepatitis, metabolic disease, immune mediated disease, genetic diseases, and others. The current understanding of the pathogenesis of fibrosis is complex, but data suggest that fibrogenesis and even cirrhosis is a dynamic process that is characterized by features of both extracellular matrix deposition and degradation. Indeed, in both animal models and in human liver disease, the evidence that fibrosis and even cirrhosis is reversal is now convincing.
Hepatitis C virus (HCV) infection has been the major cause of liver fibrosis and cirrhosis in the US for the last 20–30 years. Now, with the widespread introduction of highly effective direct acting antiviral (DAAs) drugs, many patients are now being treated, and can hope to have HCV eliminated and “cured”. An important question has become - how much fibrosis reversal should be expected after HCV elimination, both in terms of the proportion of patients who have fibrosis reversion, as well as at an individual patient level, what is the degree of fibrosis reversion expected? Additonally, in patients with cirrhosis, will the disease revert to a less severe form of cirrhosis or will the cirrhosis be eliminated? Therefore, the objective of this review will be to review the current status of the field with regard to fibrosis (and cirrhosis) reversal.
Hepatic Fibrosis – Pathophysiology
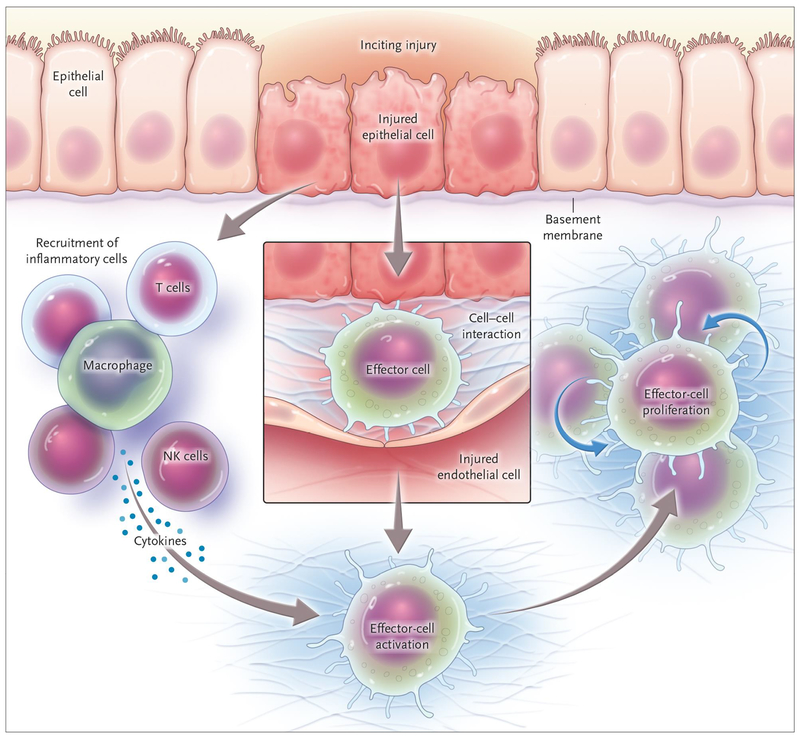
The response to recurrent injury, whether in the liver or in other organs is wound healing [1]. This wounding ressponse is highly integrated and complicated, but is characterized by 3 major features that make up the wounding mileu, including the following: 1) increased production and deposition of extracellular matrix, 2) inflammation and production of various cytokines and biologically active peptides that serve to activate effector cells, and 3), proliferation of a unique population of cells known as myofibroblasts (Figure 1). Inflammation is a common theme in most forms of chronic wound healing, and this is particularly true in HCV liver disease, where inflammation is classic and is believed to drive the fibrogenic response.
Figure 1. The wound healing response.
In essentially all forms of parenchymal injury, effector cells are activated by a complex combination of cytokines, extracellular matrix and various soluble factors. The result is fibrogenesis. (From the NEJM, Rockey et al, 2015 – reference #1)
The 3 major components of the wounding response highlighted above are integrated to lead to the final common process of enhanced extracellular matrix production, or fibrogenesis. The matrix constituents that are increased include the interstitial collagens, basement membrane collagens, proteoglycans and matrix glycoproteins such as laminin, fibronectin, including its EDA (or “cellular fibronectin”) isoforms [2]. It is also critical to emphasize that the extracellular matrix is dynamic, involving not only aspects of matrix synthesis, but also of degradation [3].
Multiple liver cell types play an important role in the hepatic wounding response. These include both primary effector cells, including peri-portal and peri-central fibroblasts, myofibroblasts, as well as other cells in the liver including hepatocytes, Kupffer cells, and even bile duct epithelial cells and endothelial cells - all of which may have cell to cell or paracrine effects on primary effector cells [1].
Altough there has been some controversy about which cell type is the most prominent fibrogenic cell, current evidence suggests that the major primary fibrogenic cell in the liver is the hepatic stellate cell [4]. Stellate cells, which are distributed throughout the hepatic lobule, are known to serve as the principal storage site for retinoids (vitamin A metabolites) and are well known for their vitamin A handling capacity [5]. A central feature of the fibrogenic response is the transformation of stellate cells from “quiescent” (normal) to an “activated” (injured liver) state [6]. The activation process is remarkably complex, and consists of many important cellular changes, the most prominent of which includes the remarkable upregulation of extracellular matrix protein synthesis, including types I, III and IV collagens, fibronectin, laminin and proteoglycans [7]. A further critical feature of activation is de novo expression of smooth muscle specific proteins, such as smooth muscle α actin [8]. This latter feature identifies stellate cells as liver specific myofibroblasts, and has important implications for the biology of wound healing [9]. While the most prominent features of stellate cell activation are the enhanced extracellular matrix production and transition to a myofibroblast cell type, activation is also associated with other cellular phenotypes including cell proliferation, contractility, release of proinflammatory cytokines, release of matrix degrading enzymes and their inhibitors, and others [6].
The factors that regulate stellate cell activation are complex and beyond the scope of this review (see [6] for detail). However, suffice it to say, that multiple factors play a key pathogenic role in stellate cell fibrogenesis. Many cytokines - in particular transforming growth factor beta-1 (TGF-β1) - appear to be critical. Peptides, including endothelin-1 (ET-1) have also been found to stimulate stellate cell activation [10]. Though numerous cytokines and peptides stimulate activation, a number of cytokines and peptides appear to have anti-activation or anti-fibrogenic properties towards stellate cells. Examples include interferon γ, interferon α, cytoglobin, and possibly adiponectin and hepatocyte growth factor. [6,11–13]. Various other factors, far too numerous to catalog here are likewise critical components of the activation process; examples include regulators of gene transcription, such as the peroxisome proliferator (PPARs), microRNAs, long non coding RNAs (lncRNAs), and many others (See [6] for Review).
Further, although it is clear that cytokines, growth factors and other soluble substances are important components of fibrogenesis. For example the ECM itself may regulate stellate cell activation, either stimulating or inhibiting it. It is also apparent that the ECM itself is dynamic, with not only synthesis, but also its degradation [14]. Indeed, during fibrosis progression, both ECM synthesis and degradation occur, with synthesis outstripping degradation. The mechanism of this ECM tunrover appears to be related to dysgreulated expression of matrix metalloproteinases (MMPs) and in particular their tissue inhibitors (TIMPs), which in aggregate favor ECM accumulation.
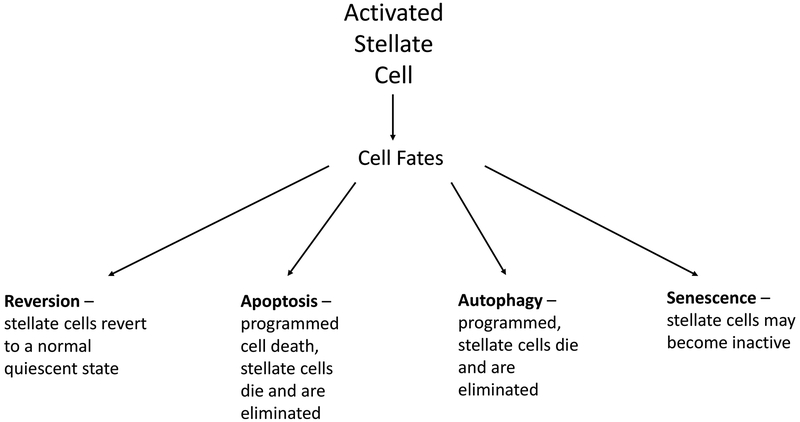
Given the importance of stellate cell activation in the pathogenesis of fibrosis, it is not suprising that mechanisms of reversion are tightly linked to the biology of stellate cell activation (Figure 2). Stellate cell activation is interrupted by any one of several processes that either lead to a new cell fate or elimination of stellate cells. These include reversion to an inactivated state, autophagy, apoptosis and perhaps even cellular senescence [15–17]. Additionally, recent evidence suggests that the immune system - in particular, macrophage populations may be important in reversion of fibrosis [18–21]. In this scenario, resident Kupffer cells and “inflammatory” macrophages secrete proinflammatory cytokines, (i.e., IL-1β and TNFα), and profibrogenic factors (TGFβ) which lead to hepatocellular injury and stimulate fibrosis. On the other hand, “restorative” macrophages (i.e., Ly-6Clow macrophages) and some resident Kupffer cells produce anti-inflammatory mediators and matrix metalloproteinases (i.e., MMP−9, −12, −13) that “quiet” the injury process, and promote degradation of extracellular matrix.
Figure 2. Stellate cell fate.
Reversal of fibrosis in the liver is linked to one of several cellular processes involving stellate cells. Stellate cells may revert to an inactive, or quiescent state (by remain primed for reactivation). They may also be eliminated via either apoptosis or autophagy. The ultimate effect of many of these processes is controversial from a cell biological standpoint, since they may actually stimulate activation of stellate cells and therefore stimulate fibrogenesis.
Fibrosis reversal
Fibrosis is reversible in many different liver diseases (Table 1). Note, for the purposes of this discussion, the terms “reversible”, “reversal”, “reversion”, and “regression” are used interchangably and are intended to indicate that the amount of fibrosis in the liver becomes reduced over time compared to the amount of fibrosis at baseline. These terms are not intended to infer or intimate that the liver returns to normal.
Table 1.
Diseases in which fibrosis may revert
| Disease | Therapy |
|---|---|
| Hepatitis B | Antivirals |
| Hepatitis C | Antivirals |
| Autoimmune hepatitis | Corticosteroids, immunosuppressives |
| Bile duct obstruction | Surgical decompression |
| Hemochromatosis | Phlebotomy (iron depletion) |
| Alcoholic hepatitis | Corticosteroids |
| Primary biliary cirrhosis | Ursodeoxycholic acid, FXR agonists |
| Non-alcoholic steatohepatitis | Lifestyle modification, obesity surgery, vit. E |
Perhaps the most robust surrounding reversibility of fibrosis (and cirrhosis) exist in patients with hepatitis B virus (HBV) infection, where inhibition of the virus leads to fibrosis and even cirrhosis reversion [22] and also clearly leads to improved outcomes [23]. Additionally, fibrosis (and cirrhosis) in patients with autoimmune hepatitis who respond to medical treatment is reversible [24]. Fibrosis also improves in patients with alcoholic liver disease who respond to anti-inflammatory therapy such as corticosteroids [25,26]. Fibrosis ws been long ago reported to revert in patients with hemochromatosis during iron depletion [27,28] and in addition, fibrosis improves after relief of bile duct obstruction [29]. In patients with non-alcoholic steatohepatitis (NASH), treatment with the peroxisomal proliferator active receptor (PPAR) gamma agonist, rosiglitazone, appears to reduce fibrosis [30].
For the purposes of this review, we will examine each fibrosis and cirrhosis, recognizing that the two entities may not always be separable and that a continuum exists. Fibrosis is typically defined histologically (for HCV, typically with Metavir or Ischak staging), though multiple studies are now reporting results with noninvasive tools (in particular, blood based assays or transient elastography).
In HCV, it is important to regcognize that the natural history of the disease without interruption in hepatic HCV infection is that fibrosis progresses over time. For example, in the HALT-C trial, which evaluated maintenance therapy with peginterferon-ribavirin, fibrosis increased substantially in the 346 nonresponders; there was an increase of 61% over pretreatment baseline after 2 years and 80% after 4 years [31]. Further, in a study of a PPAR gamma agonist (farglitizar) in Ishak stage 2–4 HCV patients, the median increase in collagen (measured by picrosirius red staining) in the placebo group was 27% over 52 weeks [32]. Thus, it is relatively clear that without interruption in HCV infection, fibrosis actively progresses over time. These also made the point that even patients with relatively advanced fibrosis (including histological cirrhosis), the natural history of the disease is for fibrosis to progress.
A number of early studies in the field, primarily performed in patients receiving interferon based therapy and using liver histological analysis of the liver, have demonstrated that fibrosis reverses in a substantial proportion of patients. In aggregate, it appears that approximately one third to nearly 50% of patients have some meaningful reduction in fibrosis stage.
A notable histological study in 38 HCV patients with cirrhosis after HCV eradication with interferon based regimens, that studied paired pre- and posttreatment liver biopsies obtained a median of 61 months after SVR, showed that the area of fibrosis decreased in 34 of 38 patients (89%), and further that there was a 72% median reduction in collagen content iin the liver [33]. Thus, this study suggests that meaningful fibrosis regression can occur.
Other studies have examined the response to therapy using non-invasive assessments. In one study, 933 patients with HCV and repeat non-invasive assessments of fibrosis (“FibroTest” and transient elastography), found that in the 415 patients with advanced fibrosis at baseline, 49% (95% CI 33–64%) of the 108 patients who had an SVR had a greater than 20% reduction in FibroTest scores, which was greater than in the 219 non-responders [23% (14–33%; p < 0.001 vs. SVR)] [34]. In those with cirrhosis, regression occurred in 24/43 patients. However it was also noted that “new cirrhosis” developed in some 15% of other patients with SVR [34].
In a study of 4731 patients with HCV infection, iin whom 1657 were treated and 755 achieved SVR; it was reported that over a 10-year period, there were significant reductions in fibrosis (assessed by FIB4) in patients who had an SVR; at 77 weeks after treatment initiation, the decrease in fibrosis score was 22% overall (P < 0.0001) [35]. The authors also reported that SVR was associated with long-term maintenance of fibrosis regression.
Patients with HCV who undergo liver transplantation represent an important subpoulation of patients with HCV in whom eradication has great implications. In one study of post transplantation HCV (with primarily patients with F0–F1 fibrosis), 38% of 116 patients with recurrence of HCV post-liver transplantation who had eradication of HCV had a dramatic reduction in fibrosis progression compared to nonresponders/relapsers [36]. Additionally, in the SVR group, 13% progressed to fibrosis stage ≥ 3 on post-treatment biopsies vs. 38% in the non-response/relapse group (P = 0.001). In another study of patients after liver transplantation, among 77 patients with advanced fibrosis who had an SVR after DAA therapy responders (27 F3, 50 F4) who underwent elastography at baseline and at the end of follow-up, 39 (51%; 18 F3, 21 F4) had a regression in fibrosis stage [37]. Finally, in another study of 112 patients with HCV recurrence after liver transplantation, there was significant regression of fibrosis in all fibrosis stages [38]. In patients with stage F3 (n = 26) and F4 (n = 37) fibrosis, 22 (85%) and 16 (43%) had a reduction in at least 1 stage of fibrosis. Additionally, in patients with cirrhosis (n = 34), liver stiffness decreased from 25.3 (16.5–32.8) to 14.5 (9.9–22.1) kPa (P < 0.001). These studies demonstrate substantial fibrosis regression in a high proportion of patients with virologic eradication post liver transplantation.
HIV-HCV coninfected patients represent another special population of HCV infection - further, these patients appear to have aggressive fibrosis. In a study of 133 HCV-HIV-coinfected patients with cirrhosis followed for a mean of 6.8 years, it was reported that fibrosis (measured by liver stiffness) appeared to regress in 23/42 (55%) of patients with an SVR, compared to 14/91(15%) of patients without an SVR, who had fibrosis regression [39]. Additonally, in a Cox survival analysis, fibrosis regression was associated with a lower risk of death (adjusted hazard ratio, HR 0.36; 95 % CI 0.15–0.86), and liver-related death (HR 0.15; 95 % CI 0.03–0.65), emphasizing the importance of fibrosis regression in HIV-HCV coinfected patients.
While the data suggest that a significant proportion of patients with HCV fibrosis, or even cirrhosis, will have significant, and apparently lasting fibrosis regression, a word of caution is important. In a study of 392 patients who received a DAA based treatment for HCV, transient elastography values recorded prior to therapy and within 18 months after therapy were evaluated [40]. In addition, Fibrosis-4 (FIB-4) index and aspartate aminotransferase-to-platelet (APRI) ratio scores were calculated. The median liver stiffness prior to DAA treatment was 12.65 kPa and decreased to 8.55 kPa post-treatment (P < 0.001), corresponding to a decline of 32.4% after DAA treatment. Median FIB-4 and APRI values significantly decreased from 2.54 and 1.10 to 1.80 and 0.43 (both P < 0.001), respectively. Another study evaluated acoustic radiation force impulse elastography, FIB-4 index, and APRI in 256 patients with chronic HCV who received pegylated interferon or DAA based therapy (85.5% achieved SVR) [41]. Paired liver stiffness values declined significantly from baseline to SVR visit in all groups and subgroups except the nonresponder subgroup (n = 10). Additionally, baseline FIB-4 (P < 0.0001) and APRI (P < 0.0001) values also declined in responders. Thus, in these studies, declines in liver stiffness measured by elastography correlated with regression of FIB-4 and APRI - the latter, validated fibrosis scores. However, declines in both of these studies were rapid, and it remains to be determined whether these changes represent true regression of fibrosis or rather resolution of necro-inflammation with subsequent improvement of liver stiffness and laboratory parameters.
Outcomes after HCV eradication
Since fibrosis regression is expected to lead to changes (perhaps improvement) in hepatocellular function, and perhaps intrahepatic blood flow (and thus portal hypertension), it follows that HCV eradication may lead to improved outcomes. In a large study of 1444 patients with a mean followup of 4.7 years, patients with sustained virological response (treated with interferon based regimens) had a lower incidence of liver-associated events compared to non-responders/relapsers/virological breakthrough, or never treated patients (1.7% vs 4.7% and 4.7%, respectively) [42]. Event-free survival was significantly higher in sustained virological response patients (P=.0082). The authors concluded that in this “real-world” cohort, the achievement of sustained virological response almost eliminated liver-related morbidity and mortality compared with patients who failed to achieve sustained virological response and those who were untreated.
In a study from 5 large tertiary care hospitals in Europe and Canada in 530 Ishak stage 4–6 patients with HCV infection treated with an interferon-based treatment regimen between 1990 and 2003, and followup for a median of 8.4 years, Cox regression analysis revealed that SVR was associated with reduced risk of all-cause mortality (hazard ratio [HR], 0.26; 95% CI, 0.14–0.49; P < .001) and reduced risk of liver-related mortality or transplantation (HR, 0.06; 95% CI, 0.02–0.19; P < .001) [43]. The 10-year cumulative incidence rate of liver-related mortality or transplantation was 1.9% (95% CI, 0.0%–4.1%) with SVR and 27.4% (95% CI, 22.0%–32.8%) without SVR (P < .001).
In another long-term study of 218 compensated cirrhotic HCV patients without esophageal varices at baseline, receiving inteferon based HCV therapy, during a median follow-up of 11.4 years, 149/218 (68%) patients received antiviral treatment and 34 (22.8%) achieved SVR [44]. No SVR patients developed varices compared with 22 (31.8%) of the 69 untreated subjects (P < 0.0001) and 45 (39.1%) of the 115 non-SVR patients (P < 0.0001). The authors In the long term, the achievement of SVR prevents the development of EV in patients with compensated HCV-induced cirrhosis.
The question as to risk of hepatocellular cancer (HCC) after HCV eradication, and fibrosis reversion is extremely complicated. A full discussion of this important topic is beyond space limitations here (see [45,46] for review).
Future
There remains much further work to be done in this field; a number of questions such as the following remain (Table 2): (1) when fibrosis reverses, does the the architecture of the liver return to normal? (2) Although it appears that fibrosis reverses in patients receiving either interferon based or DAA therapy, it is not entirely clear whether qualitative (or quantitiative) differences in fibrosis reversal exist among these 2 forms of therapy. (3) It should be noted that in general, the data are limited by relatively short durations of follow-up, and long term followup studies are needed. (4) A better understanding of which patients are most likely to exhibit reversal of fibrosis or cirrhosis is required. (5) The likelihood of complications of cirrhosis appears to be reduced after HCV eradication, but further work is required to clearly define exactly which complications are most likely and what the risk of complications is.
Table 2.
Critical questions after HCV eradication
| Does hepatic architecture return to normal after HCV elimination? |
| Is HCV eradication with an interferon based therapy the same as with a DAA based therapy in terms of fibrosis reversal? |
| Does HCV clearance lead to long term improvements in fibrosis? |
| Which patients can expect clinically significant fibrosis regression after HCV cure? |
| Assuming that fibrosis regression occurs, will this be linked to improved outcomes? |
Conclusion
Hepatic fibrosis and even cirrhosis is a dynamic process, in which matrix appears to be actively turning over. Even advanced fibrosis, at least to some extent, is reversible and currently available data now clearly indicate that elimination of HCV leads to some degree of fibrosis reversion. Since DAA’s now promise to eradicate HCV in the vast majority of patients, even in those with advanced fibrosis and cirrhosis, it will be important to continue to understand the dynamics of fibrosis reversion after SVR. Perhaps the most important area of research will be in determining which patients are most likely to expect significant fibrosis reversal. Further, what is not clear is exactly what the final result of fibrosis reversion will be. For example, will the hepatic architecture normalize or remain abnormal, or will sinsuoidal blood flow return to normal or not.
Currently, the field is too immature to make recommendations about clinical management of cirrhosis that differ from current guidelines. Thus, management of cirrhotic patients should continue to be as it currently performed, with routine liver health care maintenance, including everything from routine vaccinations to screening for hepatocellular carcinoma and esophageal varices. We are now in perhaps one of the most exciting times in hepatology – and much more data in the DAA era are anticipated in the next several years.
Key points.
With the introduction of highly effective direct acting antiviral (DAA) drugs, currently available regimens promise a nearly 100% cure rate.
After eradication of HCV, with either interferon based or DAA based regimens, fibrosis and even cirrhosis reverses in some patients.
There has been extensive new data focused on the reversibility of HCV fibrosis and cirrhosis.
Emerging data suggest improved outcomes in patients with cirrhosis after HCV clearance supports the premise that cirrhosis reverses in some patients.
Financial Support:
This work was supported by the National Institutes of Health, R01 DK 098819.
Abbreviations:
- DAA
direct acting anti-viral
- HCV
hepatitis C virus
- SVR
sustained virologic response
Footnotes
Conflict of Interest Disclosure: The author certifies that he has no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, honoraria, etc.) with a company whose product figures prominently in this manuscript or with a company making a competing product.
References
* of special interest
** of outstanding interest
- 1.Rockey DC, Bell PD, Hill JA: Fibrosis--a common pathway to organ injury and failure. N Engl J Med 2015, 372:1138–1149. [DOI] [PubMed] [Google Scholar]
- 2.Schuppan D: Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin.Liver.Dis 1990, 10:1–10. [DOI] [PubMed] [Google Scholar]
- 3.Rockey DC: Antifibrotic therapy in chronic liver disease. Clin Gastroenterol Hepatol 2005, 3:95–107. [DOI] [PubMed] [Google Scholar]
- 4.Kisseleva T, Brenner DA: The phenotypic fate and functional role for bone marrow-derived stem cells in liver fibrosis. Journal of hepatology 2012, 56:965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jophlin LL, Koutalos Y, Chen C, Shah V, Rockey DC: Hepatic stellate cells retain retinoid-laden lipid droplets after cellular transdifferentiation into activated myofibroblasts. Am J Physiol Gastrointest Liver Physiol 2018, 315:G713–G721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuchida T, Friedman SL: Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 2017, 14:397–411. [DOI] [PubMed] [Google Scholar]
- 7.Maher JJ, McGuire RF: Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J.Clin.Invest 1990, 86:1641–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockey DC, Boyles JK, Gabbiani G, Friedman SL: Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J.Submicrosc.Cytol.Pathol 1992, 24:193–203. [PubMed] [Google Scholar]
- 9.Shi Z, Rockey DC: Upregulation of the actin cytoskeleton via myocardin leads to increased expression of type 1 collagen. Lab Invest 2017, 97:1412–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shao R, Rockey DC: Effects of endothelins on hepatic stellate cell synthesis of endothelin- 1 during hepatic wound healing. J Cell Physiol 2002, 191:342–350. [DOI] [PubMed] [Google Scholar]
- 11.Rockey DC, Chung JJ: Interferon gamma inhibits lipocyte activation and extracellular matrix mRNA expression during experimental liver injury: implications for treatment of hepatic fibrosis. J Investig Med 1994, 42:660–670. [PubMed] [Google Scholar]
- 12.Shafiei MS, Shetty S, Scherer PE, Rockey DC: Adiponectin regulation of stellate cell activation via PPARgamma-dependent and -independent mechanisms. Am J Pathol 2011, 178:2690–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshizato K, Thuy le TT, Shiota G, Kawada N: Discovery of cytoglobin and its roles in physiology and pathology of hepatic stellate cells. Proc Jpn Acad Ser B Phys Biol Sci 2016, 92:77–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arthur MJ: Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol 2000, 279:G245–249. [DOI] [PubMed] [Google Scholar]
- 15.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW: Senescence of activated stellate cells limits liver fibrosis. Cell 2008, 134:657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thoen LF, Guimaraes EL, Dolle L, Mannaerts I, Najimi M, Sokal E, van Grunsven LA: A role for autophagy during hepatic stellate cell activation. J Hepatol 2011, 55:1353–1360. [DOI] [PubMed] [Google Scholar]
- 17.Troeger JS, Mederacke I, Gwak GY, Dapito DH, Mu X, Hsu CC, Pradere JP, Friedman RA, Schwabe RF: Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology 2012, 143:1073–1083 e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, Merad M, Luedde T, Trautwein C, Tacke F: Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 2009, 50:261–274. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, et al. : Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A 2012, 109:E3186–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iredale JP, Bataller R: Identifying molecular factors that contribute to resolution of liver fibrosis. Gastroenterology 2014, 146:1160–1164. [DOI] [PubMed] [Google Scholar]
- 21.Ju C, Tacke F: Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol 2016, 13:316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Schall RA, et al. : Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013, 381:468–475. [DOI] [PubMed] [Google Scholar]
- 23.Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J, et al. : A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group [see comments]. N Engl J Med 1998, 339:61–68. [DOI] [PubMed] [Google Scholar]
- 24.Dufour JF, DeLellis R, Kaplan MM: Reversibility of hepatic fibrosis in autoimmune hepatitis. Ann Intern Med 1997, 127:981–985. [DOI] [PubMed] [Google Scholar]
- 25.Ramond MJ, Poynard T, Rueff B, Mathurin P, Theodore C, Chaput JC, Benhamou JP: A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med 1992, 326:507–512. [DOI] [PubMed] [Google Scholar]
- 26.Spahr L, Rubbia-Brandt L, Pugin J, Giostra E, Frossard JL, Borisch B, Hadengue A: Rapid changes in alcoholic hepatitis histology under steroids: correlation with soluble intercellular adhesion molecule-1 in hepatic venous blood. J Hepatol 2001, 35:582–589. [DOI] [PubMed] [Google Scholar]
- 27.Powell LW, Kerr JF: Reversal of “cirrhosis” in idiopathic haemochromatosis following long-term intensive venesection therapy. Australas Ann Med 1970, 19:54–57. [DOI] [PubMed] [Google Scholar]
- 28.Blumberg RS, Chopra S, Ibrahim R, Crawford J, Farraye FA, Zeldis JB, Berman MD: Primary hepatocellular carcinoma in idiopathic hemochromatosis after reversal of cirrhosis. Gastroenterology 1988, 95:1399–1402. [DOI] [PubMed] [Google Scholar]
- 29.Hammel P, Couvelard A, O’Toole D, Ratouis A, Sauvanet A, Flejou JF, Degott C, Belghiti J, Bernades P, Valla D, et al. : Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med 2001, 344:418–423. [DOI] [PubMed] [Google Scholar]
- 30.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, et al. : Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010, 362:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman ZD, Stoddard AM, Bonkovsky HL, Fontana RJ, Ghany MG, Morgan TR, Wright EC, Brunt EM, Kleiner DE, Shiffman ML, et al. : Fibrosis progression in chronic hepatitis C: morphometric image analysis in the HALT-C trial. Hepatology 2009, 50:1738–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McHutchison J, Goodman Z, Patel K, Makhlouf H, Rodriguez-Torres M, Shiffman M, Rockey D, Husa P, Chuang WL, Levine R, et al. : Farglitazar lacks antifibrotic activity in patients with chronic hepatitis C infection. Gastroenterology 2010, 138:1365–1373, 1373 e1361–1362. [DOI] [PubMed] [Google Scholar]
- 33.D’Ambrosio R, Aghemo A, Rumi MG, Ronchi G, Donato MF, Paradis V, Colombo M, Bedossa P: A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology 2012, 56:532–543. [DOI] [PubMed] [Google Scholar]
- 34.Poynard T, Moussalli J, Munteanu M, Thabut D, Lebray P, Rudler M, Ngo Y, Thibault V, Mkada H, Charlotte F, et al. : Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. J Hepatol 2013, 59:675–683. [DOI] [PubMed] [Google Scholar]
- 35.Lu M, Li J, Zhang T, Rupp LB, Trudeau S, Holmberg SD, Moorman AC, Spradling PR, Teshale EH, Xu F, et al. : Serum Biomarkers Indicate Long-term Reduction in Liver Fibrosis in Patients With Sustained Virological Response to Treatment for HCV Infection. Clin Gastroenterol Hepatol 2016, 14:1044–1055 e1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhanasekaran R, Sanchez W, Mounajjed T, Wiesner RH, Watt KD, Charlton MR: Impact of fibrosis progression on clinical outcome in patients treated for post- transplant hepatitis C recurrence. Liver Int 2015. [DOI] [PubMed] [Google Scholar]
- 37.Martini S, Sacco M, Strona S, Arese D, Tandoi F, Dell Olio D, Stradella D, Cocchis D, Mirabella S, Rizza G, et al. : Impact of viral eradication with sofosbuvir-based therapy on the outcome of post-transplant hepatitis C with severe fibrosis. Liver Int 2017, 37:62–70. [DOI] [PubMed] [Google Scholar]; *This study demonstrated that nearly 50% of patients with post transplant HCV had a reduction in fibrosis after DAA therapy, and a similar number had a long-term improvement in liver function.
- 38.Mauro E, Crespo G, Montironi C, Londono MC, Hernandez-Gea V, Ruiz P, Sastre L, Lombardo J, Marino Z, Diaz A, et al. : Portal pressure and liver stiffness measurements in the prediction of fibrosis regression after sustained virological response in recurrent hepatitis C. Hepatology 2018, 67:1683–1694. [DOI] [PubMed] [Google Scholar]; **In patients with recurrent HCV after liver transplantation who achieved SVR (largely with IFN based therapy), 43% in recipients with cirrhosis and 72%−85% of those with other stage fibrosis had fibrosis regression. HVPG was also reduced after SVR.
- 39.Casado JL, Esteban MA, Banon S, Moreno A, Perez-Elias MJ, Mateos ML, Moreno S, Quereda C: Fibrosis Regression Explains Differences in Outcome in HIV-/HCV-Coinfected Patients with Cirrhosis After Sustained Virological Response. Dig Dis Sci 2015. [DOI] [PubMed] [Google Scholar]
- 40.Bachofner JA, Valli PV, Kroger A, Bergamin I, Kunzler P, Baserga A, Braun D, Seifert B, Moncsek A, Fehr J, et al. : Direct antiviral agent treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers fibrosis-4 score and aspartate aminotransferase-platelet ratio index. Liver Int 2017, 37:369–376. [DOI] [PubMed] [Google Scholar]; **In a large cohort of patients treated with DAAs, this study demonstrated that there is a rapid decrease in TE that parallels changes in FIB-4 and APRI fibrosis scores. Because of the rapid decrease, this study raises the possibility that this represents merely resolution of chronic liver inflammation with subsequent improvement of TE values and laboratory parameters (rather than a change in fibrosis).
- 41.Chen SH, Lai HC, Chiang IP, Su WP, Lin CH, Kao JT, Chuang PH, Hsu WF, Wang HW, Chen HY, et al. : Changes in liver stiffness measurement using acoustic radiation force impulse elastography after antiviral therapy in patients with chronic hepatitis C. PLoS One 2018, 13:e0190455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wedemeyer H, Reimer J, Sandow P, Hueppe D, Lutz T, Gruengreiff K, Goelz J, Christensen S, Pfeiffer-Vornkahl H, Alshuth U, et al. : Long-term outcome of chronic hepatitis C virus infection in a real-world setting: The German LOTOS study. Liver Int 2017, 37:1468–1475. [DOI] [PubMed] [Google Scholar]; *The LOTOS cohort highlights the importance of timely and effective treatment for patients with HCV (in this study with interferon based therapy), demonstrating an essential elimination of liver related morbidity and mortality compared with patients who fialed to achieve SVR or who were not treated.
- 43.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, Heathcote EJ, Manns MP, Kuske L, et al. : Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA : the journal of the American Medical Association 2012, 308:2584–2593. [DOI] [PubMed] [Google Scholar]
- 44.Bruno S, Crosignani A, Facciotto C, Rossi S, Roffi L, Redaelli A, de Franchis R, Almasio PL, Maisonneuve P: Sustained virologic response prevents the development of esophageal varices in compensated, Child-Pugh class A hepatitis C virus-induced cirrhosis. A 12-year prospective follow-up study. Hepatology 2010, 51:2069–2076. [DOI] [PubMed] [Google Scholar]
- 45.Ioannou GN, Green PK, Beste LA, Mun EJ, Kerr KF, Berry K: Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J Hepatol 2018, 69:1088–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]; **In this study of 45,810 patients who initiated antiviral treatment in the Veterans Affairs (VA) national healthcare system from 1/1/2009 to 12/31/2015, including both interferon based and DAA based regimens, it was shown that patients having an SVR had significant reductions in the risk of developing HCC.
- 46.Ioannou GN, Feld JJ: What Are the Benefits of a Sustained Virologic Response to Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection? Gastroenterology 2019, 156:446–460 e442. [DOI] [PubMed] [Google Scholar]; *This review of currently available evidence highlights the potential benefits of achieving SVR in different clinical settings, and emphasizes the reduction in the risk of development of HCC after SVR.