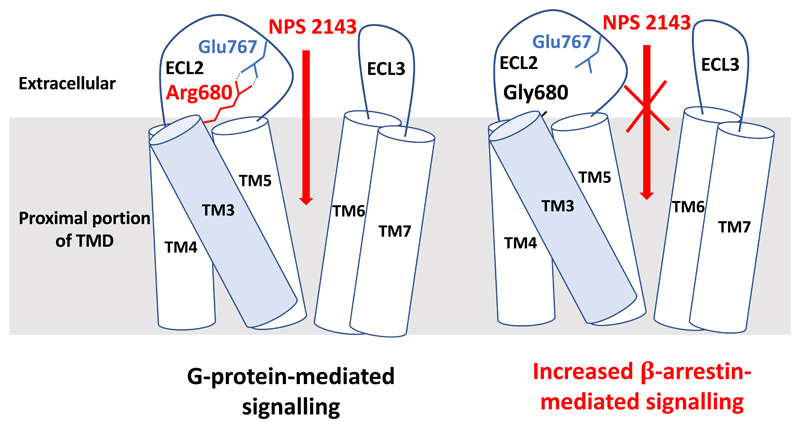
Figure 3. Disruption of a salt-bridge within the CaSR TMD causes biased signalling.
The salt-bridge is formed by the Arg680 residue located in the proximal portion of transmembrane helix 3 (TM3, shaded blue) and the Glu767 residue located in extracellular loop 2 (ECL2). The Arg680-Glu767 salt-bridge is situated at the entrance of the allosteric modulator binding pocket, which is formed by residues from TM3 and TM5-TM727. The Arg680 residue mediates the binding of the NPS 2143 calcilytic compound27. The Arg680-Glu767 salt-bridge is associated with G-protein-mediated signalling, whereas disruption of the salt-bridge by the ADH-causing CaSR mutation, Arg680Gly, selectively increases β-arrestin-mediated signalling, as well as abrogating the inhibitory effect of the NPS 2143 compound51.