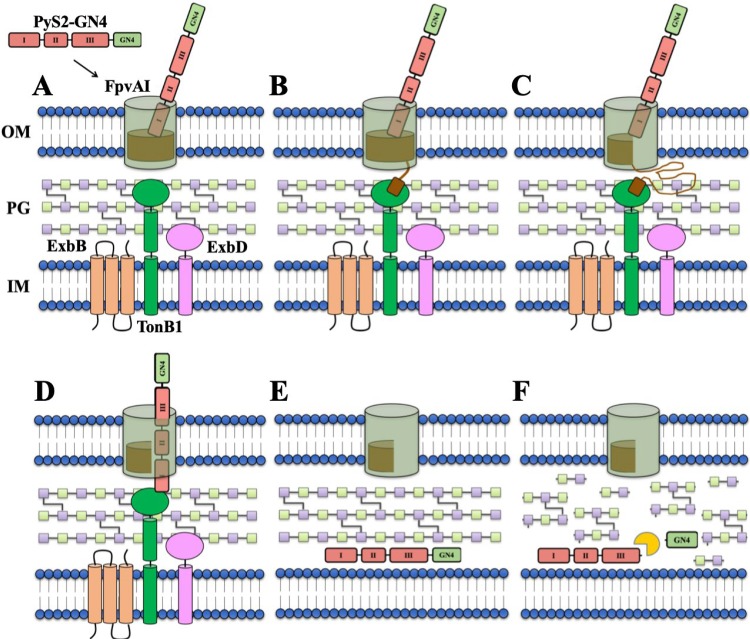
FIG 6.
Schematic overview of the proposed mechanism of PyS2-GN4 antipseudomonal activity. (A) When added extrinsically as a purified recombinant protein, domain I of PyS2-GN4 binds to the FpvAI receptor located on the surface of target P. aeruginosa bacteria. (B) This protein-protein interaction induces a conformational change in the receptor structure, resulting in the FpvAI TBB in the periplasm to recruit and bind TonB1. (C) The formation of this complex allows for the PMF-dependent unfolding of the labile half of the FpvAI plug domain. (D) Next, the unstructured region of lysocin domain I passes through the channel created in order to enable its own TBB to bind another nearby TonB1 protein in the periplasm. (E) The newly formed lysocin-TonB1 translocon stimulates the PMF-driven unfolding and import of the remainder of the lysocin into the periplasm. (F) Upon refolding, GN4 is proteolytically released and cleaves the PG to provoke partial membrane destabilization, cytoplasmic leakage, PMF disruption, and cell death.