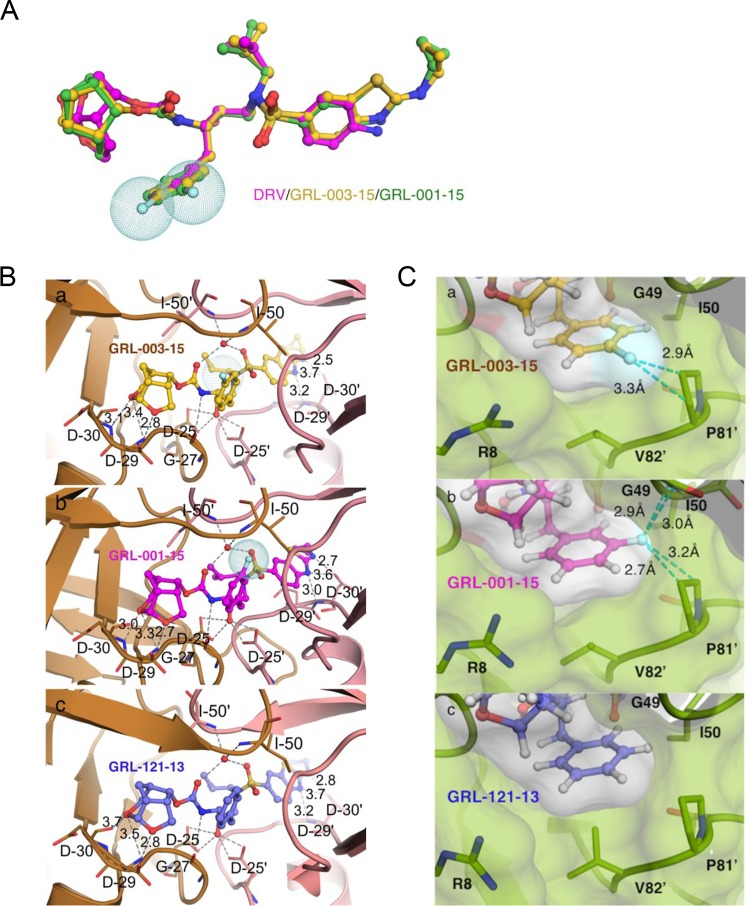
FIG 3.
X-ray crystal structure of GRL-003-15 and GRL-001-15 in the active site of wild-type HIV-1 protease. (A) Structure of DRV (magenta) overlaid with those of GRL-003-15 (yellow) and GRL-001-15 (green). All three compounds were superimposed, built on the same scaffold in the central part around the sulfonyl moiety. Note that in addition to fluorine atoms (shown as light blue-dotted spheres) attached to the P1-benzene ring, GRL-003-15 and GRL-001-15 have two moieties, P2-Crn-THF and P2′-Cp-Abt, that are larger than those of DRV. (B) Carbon atoms of GRL-003-15 (a), GRL-001-15 (b), and GRL-121-13 (c), with substitutions of HIV-PRWT shown in brown tones. Nitrogen, oxygen, sulfur, and fluorine atoms are shown in blue, red, yellow, and cyan, respectively. Hydrogen bond interactions of each compound with protease residues in the active site are shown by gray dashed lines. (C) Close look at fluorine atom interactions of GRL-121-13 (a), GRL-003-15 (b), and GRL-001-15 (c) inside the S1 subpocket of HIV-PRWT. Essential fluorine-mediated interactions are shown with cyan dashed lines. The para-positioned fluorine from GRL-003-15, however, interacts exclusively with the P81′ ring. The meta-positioned fluorine from GRL-001-15 engages in halogen interactions with main chain of G49 as well as the P81′ ring. The P1 ring of GRL-121-13 does not form any interactions. Numbers with dashed lines indicate the distance (Å) of the molecular interaction.