Invasive collection methods are often required to obtain samples for the microbiological evaluation of children with presumptive pulmonary tuberculosis (PTB). Nucleic acid amplification testing of easier-to-collect stool samples could be a noninvasive method of diagnosing PTB.
KEYWORDS: Xpert MTB/RIF assay, childhood TB, pediatric infectious disease, pulmonary tuberculosis, stool
ABSTRACT
Invasive collection methods are often required to obtain samples for the microbiological evaluation of children with presumptive pulmonary tuberculosis (PTB). Nucleic acid amplification testing of easier-to-collect stool samples could be a noninvasive method of diagnosing PTB. We conducted a systematic review and meta-analysis to evaluate the diagnostic accuracy of testing stool with the Xpert MTB/RIF assay (“stool Xpert”) for childhood PTB. Four databases were searched for publications from January 2008 to June 2018. Studies assessing the diagnostic accuracy among children of stool Xpert compared to a microbiological reference standard of conventional specimens tested by mycobacterial culture or Xpert were eligible. Bivariate random-effects meta-analyses were performed to calculate pooled sensitivity and specificity of stool Xpert against the reference standard. From 1,589 citations, 9 studies (n = 1,681) were included. Median participant ages ranged from 1.3 to 10.6 years. Protocols for stool processing and testing varied substantially, with differences in reagents and methods of homogenization and filtering. Against the microbiological reference standard, the pooled sensitivity and specificity of stool Xpert were 67% (95% confidence interval [CI], 52 to 79%) and 99% (95% CI, 98 to 99%), respectively. Sensitivity was higher among children with HIV (79% [95% CI, 68 to 87%] versus 60% [95% CI, 44 to 74%] among HIV-uninfected children). Heterogeneity was high. Data were insufficient for subgroup analyses among children under the age of 5 years, the most relevant target population. Stool Xpert could be a noninvasive method of ruling in PTB in children, particularly those with HIV. However, studies focused on children under 5 years of age are needed, and generalizability of the evidence is limited by the lack of standardized stool preparation and testing protocols.
INTRODUCTION
At least 1 million incident tuberculosis (TB) cases and 230,000 TB-related deaths are estimated to have occurred among children in 2017, accounting for approximately 10% of total cases and 15% of deaths (1). Pulmonary TB (PTB) is the most common form of childhood TB (2). Xpert MTB/RIF (Xpert) (Cepheid, USA), an automated cartridge-based PCR assay, is currently recommended by the World Health Organization (WHO) as the initial diagnostic test in presumptive PTB cases for adults and children (3). Minimal sample preparation is required, and test results are produced within 2 h. In a meta-analysis that pooled data from sputum smear-positive and -negative subjects, the performance of Xpert on respiratory samples had a sensitivity of 62% (95% credible interval, 51 to 73%) and a specificity of 98% (95% credible interval, 97 to 99%). The use of Xpert on sputum is thus more sensitive than smear microscopy. Moreover, Xpert has several operational advantages over mycobacterial culture, the gold standard for TB diagnosis (4). However, in children under 5 years old, and particularly in those under 2 years old, the collection of sputum specimens is difficult and often requires invasive methods that are challenging to implement in resource-limited settings (e.g., nasopharyngeal/nasogastric aspiration or bronchoscopy) and not widely available (2). Furthermore, as pediatric TB is typically paucibacillary, the sensitivity of currently deployed tests is diminished in children versus adults (5).
Mycobacterium tuberculosis-containing sputum may be swallowed, particularly during sleep, and acid-fast bacilli have been shown to survive digestion and are detectable in stool (6, 7). As such, stool may represent a more acceptable and feasible alternative to conventional specimens for the evaluation of suspected childhood PTB. The use of Xpert on stool has not been included in recommendations by the WHO, nor has any claim been made by the manufacturer regarding stool. However, several groups have now developed preprocessing methods in order to use Xpert on stool for the diagnosis of childhood TB.
We performed a systematic review and meta-analysis of the diagnostic performance of Xpert using stool samples for PTB in children.
MATERIALS AND METHODS
Protocol and registration.
The protocol for this systematic review and meta-analysis was registered at the International Prospective Register of Systematic Reviews (PROSPERO) (identifier CRD42017079836).
Search strategy and information sources.
PubMed, EMBASE, Scopus, and the Cochrane Library were systematically searched from 1 January 2008 until 15 June 2018. The search strategy was developed with a medical librarian and based on key validated terms for “children” and “Xpert,” as well as “tuberculosis,” with no filters applied. The full search strategies for each database are presented in Text S1 in the supplemental material. Experts in TB diagnostics were consulted to identify relevant papers that may have been missed by the search strategy. Citations of reviews and included publications were also searched.
Eligibility criteria.
Publications in English, French, Italian, Mandarin, Spanish, and Portuguese; of any design and sampling strategy; and of any enrollment timing (prospective, retrospective, or cross-sectional) were eligible for inclusion. Conference proceedings and abstracts, commentaries, editorials, and reviews were excluded, as were studies with a sample size of less than 10. To be included, eligible studies must have reported the diagnostic performance of stool Xpert in patients under 16 years old, compared to a microbiological reference standard for the diagnosis of PTB. Studies that did not explicitly state that their focus was PTB were eligible if the types of specimens used for the reference standard were those that are typically used for PTB diagnosis (e.g., gastric aspirate). Studies that used banked sputum and stool specimens originally collected from children were also eligible.
Study screening and selection.
Search results were imported into a citation manager, and duplicates were removed. Two authors (E. MacLean and G. Sulis) independently screened citations by title and abstract per predefined eligibility criteria, followed by full-text review for all selected studies. Results disagreed upon were discussed, and a third reviewer consulted if necessary (F. Ahmad Khan).
Data extraction.
A data extraction form was piloted by two reviewers (E. MacLean and G. Sulis) with critical input from a third (C. M. Denkinger). Two reviewers (E. MacLean and G. Sulis) independently extracted results from all included studies using a standardized form (Text S2). After data extraction, results were compared, and disagreements were discussed until a consensus was reached. Study authors were contacted for missing performance data, clarification regarding reference standard definitions, and sample preparation techniques. Using these data and figures indicated in the publications, we reconstructed two-by-two tables for stool Xpert performance compared to the microbiological reference standard and, where applicable, the clinical reference standard.
Risk-of-bias assessment.
The Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool (8) was used to assess each included study’s risk of bias. No formal assessment of publication bias was made, as traditional methods such as funnel plots and regression tests are not helpful for diagnostic studies (9).
Reference standards.
Acceptable microbiological reference standards were mycobacterial culture or Xpert MTB/RIF, performed on specimens that are conventionally used to diagnose childhood PTB (nasogastric aspirates, gastric lavage fluid, nasopharyngeal aspirates, and expectorated sputum). No studies included stool mycobacterial culture in their diagnostic workup. Stool Xpert was not included in the reference standard.
Childhood PTB is often clinically diagnosed (i.e., without microbiological confirmation). As such, we also examined the performance of stool Xpert compared to clinical reference standards that are compatible with updated international guidelines (5). Studies that followed these guidelines used a combination of signs and symptoms, chest radiography, epidemiological history, and tuberculin skin test (TST) results to classify children as “likely TB,” “unconfirmed TB,” and “unlikely TB” (Table S1). For our purposes, we dichotomized these outcomes into “likely/possible TB” and “unlikely TB.”
Statistical analysis.
Data from reconstructed two-by-two tables were used to calculate sensitivity and specificity and the associated 95% confidence intervals (CIs). In cases of empty cells in two-by-two tables, a zero correction was made by replacing the cell with 0.5. Aggregate-data meta-analyses were performed with bivariate random-effect hierarchical models (10) to estimate pooled sensitivity and specificity for stool Xpert compared to the microbiological reference standard and, separately, compared to the clinical reference standard. We also estimated pooled sensitivity and specificity stratified by HIV status. Results from individual studies and pooled estimates are presented on forest plots. To assess between-study heterogeneity, we used the I2 statistic (11). In a sensitivity analysis, we estimated pooled sensitivity and specificity after excluding studies that used Xpert MTB/RIF but not mycobacterial culture of conventional specimens as the microbiological reference standard. All analyses were conducted using the Midas package in STATA (STATA 15; Stata Corp., USA) (12). The study is reported according to PRISMA guidelines (Table S2) (13).
RESULTS
Search results.
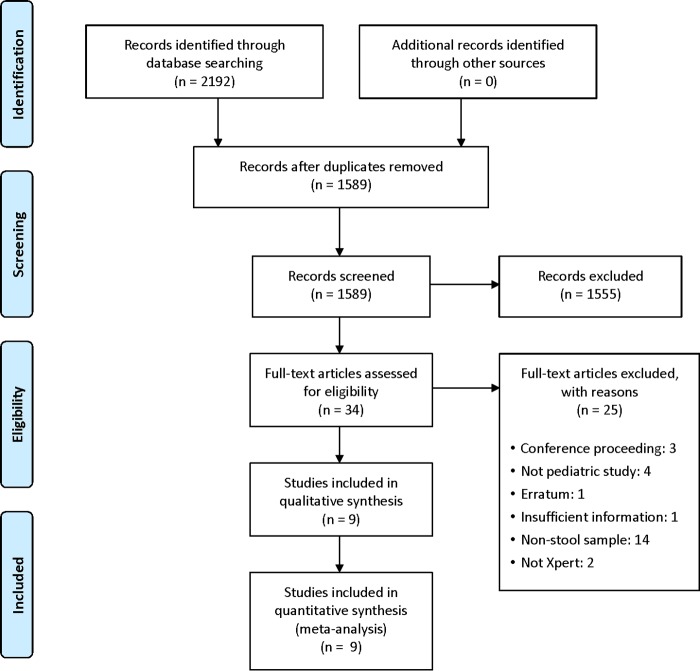
Our search identified 1,589 unique citations from which 34 studies were selected for full-text review, and 9 studies met inclusion criteria (Fig. 1).
FIG 1.
PRISMA study flow diagram.
Study and participant characteristics.
Study and patient characteristics are presented in Table 1. Among the 9 studies that we included, African countries were most well represented (7/9), whereas 2 studies recruited participants from Asia. One study had multiple sites across two continents, whereas the others were single-country studies. In total, 1,681 children from 9 studies were included in our meta-analysis of stool Xpert’s diagnostic performance compared to a microbiological reference standard, and 869 children from 5 studies were included in the comparison against a clinical reference standard. The prevalence of microbiologically confirmed cases per study ranged widely, from 2.6% (14) to 54% (15). The prevalence of clinically confirmed or unconfirmed cases was much higher, ranging from 35% (16) to 100% (17). Table S1 in the supplemental material provides details on clinical reference standard definitions of the included studies.
TABLE 1.
Features of included studies and participantsg
| Study authors, yr (reference) | Location(s) | No. of eligible children | Age range, median (yr) (IQR) | No. of patients/total no. of patients (%) |
Clinical features reported | EPTB status (no. of patients with EPTB [%]) | Reference standard | Sample type(s) used for reference standard | Total no. of specimens included in analysis | No. of microbiologically confirmed cases (%) | No. of clinically confirmed/unconfirmed cases (%) | No. of cases of clinically unlikely TB (%) | No. of contaminated cultures (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | TB history | TB contact history | TST positive | HIV positive | |||||||||||||
| Banada et al., 2016 (15) | South Africa | 40 | 0–15, NR | 21/38 (55) | NR | 16/38 (42) | NR | 16/38 (42) | Cough, EP symptoms, wt loss | PTB only | Xpert | IS, GA | 37 | 20 (54) | NR | ||
| Chipinduro et al., 2017 (17) | Zimbabwe | 218 | 5–16, 10.6 (8–13) | 123/218 (56) | 17/218 (7.8) | 51/218 (23) | NR | 111/198 (56) | Cough, wt loss, night sweats, fever, appetite loss | PTB onlya | Culturec /Xpert | IS | 218 | 19 (8.7) | NR | ||
| CRSb | 32 | 32 (100) | 0 (0) | NR | |||||||||||||
| Hasan et al., 2017 (16) | Pakistan | 50 | 0–15, 6.8 (2–9) | 22/50 (44) | NR | 27/50 (54) | NR | 0/50 (0) | Cough, EP symptoms, wt loss | PTB only | Cultured /Xpert | Sputum, GA | 49 | 11 (22) | NR | ||
| CRSb | 49 | 17 (35) | 32 (65) | NR | |||||||||||||
| LaCourse et al., 2018 (18) | Kenya | 165 | 0–12, 2 (1.1–4.8) | 75/165 (45) | NR | 20/162 (12) | 7/151 (4.6) | 165/165 (100) | Cough, lethargy, fever, failure to thrive | PTB onlya | Culturef /Xpert | Sputum, GA | 147 | 11 (7.5) | NR | ||
| CRSb | 165 | 85 (52) | 80 (48) | NR | |||||||||||||
| Marcy et al., 2016 (20) | Burkina Faso, Cambodia, Cameroon, Vietnam | 272 | 0–13, 7.2 (4.1–7.2) | 132/272 (49) | 49/272 (18) | 58/272 (21) | 50/272 (18) | 272/272 (100) | Cough, wt loss, lethargy, fever, broad-spectrum Abx failure, CXR abnormality | PTB onlya | Culturee | GA, IS, NS, string | 272 | 27 (10) | NR | ||
| CRSb | 272 | 245 (90) | 27 (10) | NR | |||||||||||||
| Moussa et al., 2016 (21) | Egypt | 115 | 1–16, NR | 45/115 (39) | NR | 29/115 (25) | 13/67 (19) | 0/115 (0) | Cough, wt loss, night sweats, fever, CXR abnormality | PTB only | Culturec | Sputum, IS | 115 | 36 (31) | 0/115 (0) | ||
| Nicol et al., 2013 (22) | South Africa | 115 | 1–15, 2.6 (1.6–4.8) | NR | 0/115 (0) | NR | NR | 17/115 (15) | Cough, wt loss, CXR abnormality | PTB only | Cultured | IS | 115 | 17 (15) | NR | ||
| Orikiriza et al., 2018 (14) | Uganda | 357 | 1–14, NR | 178/392 (45) | 8/392 (2.0) | 76/391 (19) | 99/383 (26) | 121/388 (31) | Cough, wt loss, night sweats, lethargy, fever | PTB onlya | Culturee /Xpert | Sputum, IS | 349 | 9 (2.6) | 6/357 (1.7) | ||
| Walters et al., 2017 (19) | South Africa | 379 | 0–13, 1.3 (0.8–2.4) | 184/379 (49) | 27/379 (7.1) | 214/379 (56) | 82/294 (28) | 51/379 (13) | Cough, wt loss, fever | Mix of EPTB and PTB (35/379 [9.2]) | Cultured /Xpert | GA, IS, NA, string | 379 | 72 (19) | NR | ||
| CRSb | 351 | 242 (69) | 109 (31) | NR | |||||||||||||
Implied only pulmonary TB cases based on collection of respiratory samples only.
Definitions of each clinical reference standard are given in Table S1 in the supplemental material.
Lowenstein-Jensen solid culture.
Bactec MGIT liquid culture.
Both Lowenstein-Jensen solid cultures and MGIT liquid culture.
MGIT liquid culture, with positive samples then being subcultured on Lowenstein-Jensen medium for 3 additional weeks.
Some studies included separate comparisons of stool Xpert for microbiological and clinical reference standards. Abbreviations: Abx, antibiotics; CRS, clinical reference standard; CXR, chest X ray; EP, extrapulmonary; EPTB, extrapulmonary TB; GA, gastric aspirate; IQR, interquartile range; IS, induced sputum; NA, nasopharyngeal aspirate; NR, not reported; TST, tuberculin skin test.
Studies enrolled children from 0 to 16 years of age. The ratio of females to males was generally balanced. The percentage of participants with a documented history of TB disease contact, when reported (5/9 studies), ranged from 12% (18) to 56% (19). Most studies did not include information about tuberculin skin test (TST) results. Two studies included only children with HIV (18, 20), and two restricted enrollment to HIV-negative children (16, 21); the remainder had a mixed population.
Sample processing.
Table 2 shows the sample preparation steps utilized in each study. In one study (19), two sample preparation methods were attempted, with results ultimately being pooled. Most studies (6/9) obtained one stool sample from enrolled children, typically within 24 h of obtaining respiratory samples. Samples were either used immediately or stored for later use, except for one study (20) which used some samples immediately and some after freezing and a second study (19) which stored samples collected at the child’s home and immediately used those collected at the health care center. As information on sample storage was not available for all studies, subgroup analysis could not be performed per sample storage method.
TABLE 2.
Details of stool sample storage and processing for each of the included studiesa
| Study authors, yr (reference) | No. of samples collected, mass (g) | Stool sample collection timing | Immediate use | Storage method | Stool mass used for Xpert (g) | First reagent(s) added to stool | Homogenization method(s) | Duration of specimen settling | Additional reagent(s) and or filtering/processing procedure(s) | Pellet processing procedure | Final sample loaded into cartridge |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Banada et al., 2016 (15) | 1, 5 | NR | No | 4°C for 7 days | 0.6 | 2 ml processing buffer (AL buffer, 10% povidone), 2 ml Xpert buffer | Vortexing with glass beads | 30 min at RT | All syringe filtered | No pellet | 2 ml added to cartridge |
| Chipinduro et al., 2017 (17) | 1, 5 | Within 24 h of respiratory sample collection | No | 4°C for max of 2 days | 0.15, using sterile loop | 2.4 ml PBS | Vortexing | 20 min at RT | 1 ml supernatant taken, centrifuged at 3,200 rpm for 15 min | Pellet resuspended in 1 ml PBS | Diluted 2:1 in buffer, added to cartridge |
| Hasan et al., 2017 (16) | 1, NR | Within 24 h of respiratory sample collection | No | 2–8°C (days NR), taken to tertiary hospital, stored at −80°C | 0.15 | 2.4 ml PBS | Vortexing | 20 min at RT | 1 ml supernatant taken, centrifuged at 3,500 rpm for 15 min | Pellet resuspended in 1 ml PBS | Diluted 2:1 in buffer, added to cartridge |
| LaCourse et al., 2018 (18) | 1, 2–15 | Within 24 h of respiratory sample collection | Yes | NA | NR | Equal vol of PBS | Manual homogenization | 12 to 48 h at 2–5°C | All filtered through fine filter, vortexed; added to equal vol of NaOH-NALC; PBS (concn NR) added to 40 ml and centrifuged twice | Pellet resuspended in 1.4 ml PBS by vortexing | Diluted 2:1 in buffer, added to cartridge |
| Marcy et al., 2016 (20) | NR, 0.5 | NR | Both | Some frozen (temp and days NR) | 0.5 | 10 ml Sheather’s solution (28% sucrose) | Manual homogenization, vortexing for 30 s | NR | All filtered through funnel gauze; centrifuged at 100 × g for 1 min | No pellet | 0.5 ml supernatant, 1.8 ml buffer added to cartridge; mixture allowed to sit for 15 min at RT; shaken; run |
| Moussa et al., 2016 (21) | 2, 2 | NR | Yes | NA | 2 | 10 ml distilled H2O | Vortexing | NR | Supernatant (concn NR) taken, centrifuged at 4,000 rpm for 20 min | Pellet decontaminated in 10 ml 3% NALC-NaOH for 15 min at RT; added to 40 ml PBS; centrifuged for 20 min; pellet resuspended in 1 ml PBS | Diluted 2:1 in buffer, added to cartridge |
| Nicol et al., 2013 (22) | 1, NR | At baseline | No | −80°C within 2 h for max of 6 mo | 0.15 using FLOQ swabs | 2.4 ml PBS | Vortexing | 20 min at RT | 1 ml supernatant taken, centrifuged at 3,200 rpm for 15 min | Pellet resuspended in 1 ml PBS | Diluted 2:1 in buffer, added to cartridge |
| Orikiriza et al., 2018 (14) | 1, NR | NR | Yes | NA | NR | Saline solution | Vortexing | 5 min at RT | 5 ml mixture taken, added to NaOH-NALC, vortexed, with standing for 20 min; PBS added to 50 ml and centrifuged at 3,000 × g for 20 min at 4°C | Pellet decontaminated with NaOH-NALC method, respun; pellet resuspended in 1.5 ml unspecified buffer | 0.5 ml added to cartridge |
| Walters et al., 2017 (19) | 1, 0.3–5 | Within 7 days of respiratory sample collection | Both | 2–8°C for max of 3 days if collected at home | <5 | 20 ml PBS | Vortexing | None | 5 ml mixture taken, added to NALC-NaOH | Concentration | Diluted 2:1 in buffer, added to cartridge |
| 1, 0.3–5 | Within 7 days of respiratory sample collection | Both | 2–8°C for max of 3 days if collected at home | 1–4 | 10 ml PBS | Vortexing | None | All centrifuged at 3,000 × g at 4°C for 20 min | Pellet resuspended in 10 ml by vortexing for 20 s; centrifuged at 2,000 × g for 1 s; supernatant kept | 1 ml supernatant added to cartridge |
Abbreviations: max, maximum; NA, not applicable; NR, not reported; PBS, phosphate-buffered saline; RT, room temperature; NALC-NaOH, N-acetyl-l-cysteine–sodium hydroxide.
The mass of stool utilized, and its collection method, varied: 0.15 g of bulk stool (16), 0.15 g using a sterile loop (17), a flocked rectal swab (22), 0.5 g (21), 0.6 g (15), 2 g (20), and 5 g (19). A diluent solution, such as phosphate-buffered saline (PBS), distilled water, or a sucrose solution, was added to the stool before homogenization, in various quantities, typically followed by vortexing. Most studies (6/9) reported a period of sample settling before further workup. Final sample preparation methods were quite varied but included either centrifugation or filtering through a syringe filter or gauze, primarily to remove large particles, before final addition of the sample to the Xpert cartridge (Table 2).
Quality assessment.
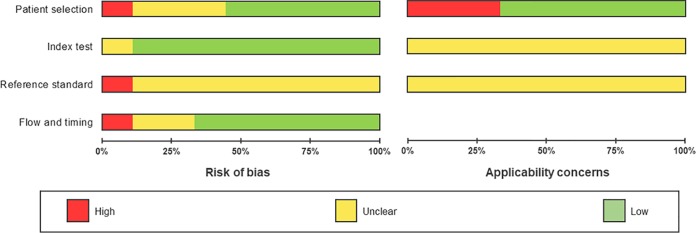
Figure 2 displays the overall risk of bias and applicability concerns of the 9 studies included in our meta-analysis. Figure S1 presents the individual studies’ quality assessment results. In the patient selection domain (Fig. 2), five studies were at low risk of bias, and one study (15) was at high risk of bias due to its use of a case-control design, whereas the remaining eight were either cross-sectional or cohort studies. Risk of bias was high for one study because of convenience sampling (16) and unclear in two studies because of an unclear sampling strategy and inappropriate exclusions of certain children (17, 21). With respect to applicability, the majority of studies (Table 1) included children who presented with symptoms suggestive of TB. Two studies (18, 20) included only children with HIV, and because it is known that Xpert performs differentially for those who are HIV infected (23), these studies were scored for applicability concerns as high. One study (15) tested only samples from confirmed TB cases and noncases, which does not represent a typical clinical scenario, so we also rated applicability concerns as high.
FIG 2.
QUADAS-2 risk of bias and applicability concerns graph. The review authors’ judgements about each domain are presented as percentages across the 9 included studies.
The conduct of the index test generally was at low risk of bias, as Xpert is an automated assay with a predefined cutoff of detection that produces a binary response. However, since there is no standardized operating protocol for stool samples and no internationally recommended procedure for sample storage and processing, applicability concerns regarding the index test’s conduct are unclear (Fig. 2).
In light of the inherent limitations of microbiological tests for diagnosing childhood PTB, we classified 8/9 studies as having an unclear risk of bias with respect to correctly classifying the target condition despite having used culture as the reference test. The exception was one study that was scored as having a high risk of bias as its microbiological reference standard did not include culture. Both culture and Xpert are automated assays, so we scored the risk of bias as low regarding test result interpretation. Additionally, all studies’ reference standards were performed in regional or central reference laboratories, so we expect bias from operator error to be of low concern. Applicability concerns were uniformly unclear.
We scored the risk of bias as low for all studies with respect to the appropriateness of the time interval between the index test and the reference standard, as all studies reported running stool Xpert within 7 days of specimen collection (Fig. 2).
Meta-analysis of diagnostic accuracy.
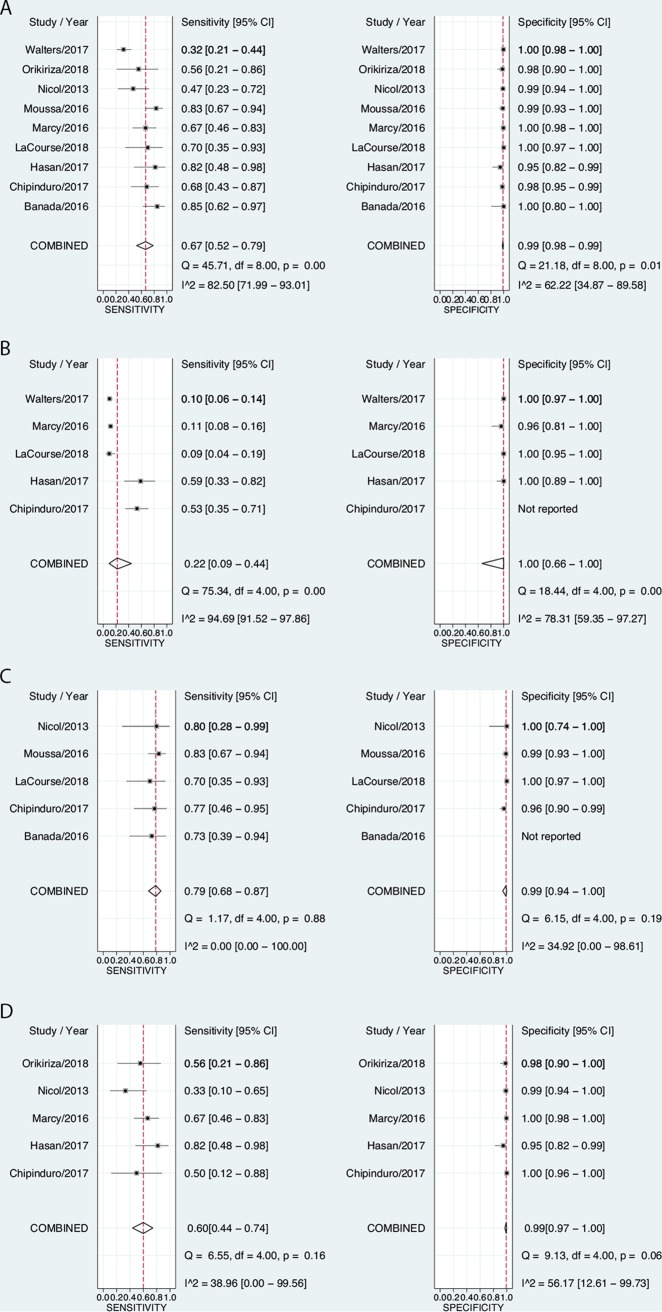
For comparison against the microbiological reference standard, sensitivities of stool Xpert varied from 32% (19) to 85% (15), while specificity was uniformly very high (Fig. 3A). The pooled sensitivity was 67% (95% CI, 52 to 79%), and the pooled specificity was 99% (95% CI, 98 to 99%). I2 values for sensitivity and specificity were 83% (95% CI, 72 to 93%) and 62% (95% CI, 35 to 90%), respectively, indicating high between-study heterogeneity, particularly for sensitivity. For the clinical reference standard comparison, the pooled sensitivity of stool Xpert was 22% (95% CI, 9.0 to 44%), while the specificity was 100% (95% CI, 66 to 100%) (Fig. 3B).
FIG 3.
(A) Forest plots of stool Xpert’s diagnostic performance compared to a microbiological reference standard of culture or Xpert positivity on respiratory samples (14–22). Two studies (18, 20) presented results from “intention-to-treat” (ITT) analyses, where any child who produced any sample was included, as well as “per-protocol” analyses, where only children who produced all requested samples were included. In these instances, we meta-analyzed the ITT results to avoid selection bias. (B) Forest plots of stool Xpert’s diagnostic performance compared to a clinical reference standard of “likely/possible TB” or “unlikely TB.” (C) Forest plots of diagnostic performance of stool Xpert in children with HIV compared to a microbiological reference standard. (D) Forest plots of diagnostic performance of stool Xpert in HIV-negative children compared to a microbiological reference standard.
Although 7/9 studies included children with HIV, only 5/9 studies provided sufficient information to construct two-by-two tables (15, 17, 18, 21, 22) (2 of these studies enrolled only children with HIV [18, 21]) (Fig. 3C). One study (15) did not provide sufficient information to calculate specificity among children with HIV. Data from children who were HIV negative were available from 5 studies (14, 16, 17, 20, 22) (Fig. 3D). Using the microbiological reference standard, among children with HIV, the sensitivity of stool Xpert was 79% (95% CI, 68 to 87%), and the pooled specificity was 99% (95% CI, 94 to 100%) (Fig. 3C); among those without HIV, the sensitivity was 60% (95% CI, 44 to 79%), and the specificity was 99% (95% CI, 97 to 100%) (Fig. 3D). For both sensitivity and specificity, I2 values were lower in HIV-stratified analyses than when data from all studies were pooled (Table 3), suggesting that HIV partially explained the between-study heterogeneity.
TABLE 3.
Results of meta-analyses for estimated stool Xpert sensitivity and specificitya
| Comparison | Main results |
Results of sensitivity analysis excluding the study that did not use culture as reference standard |
||||
|---|---|---|---|---|---|---|
| No. of studies included (no. of children included) | Pooled sensitivity (%) (95% CI); I2 statistic (95% CI) | Pooled specificity (%) (95% CI); I2 statistic (95% CI) | No. of studies included (no. of children included) | Pooled sensitivity (%) (95% CI); I2 statistic (95% CI) | Pooled specificity (%) (95% CI); I2 statistic (95% CI) | |
| Stool Xpert against microbiological reference standard | 9b (1,681) | 67 (52–79); 83 (72–93) | 99 (98–99); 62 (35–90) | 8f (1,644) | 64 (49–76); 81 (69–93) | 99 (98–100); 61 (31–91) |
| Stool Xpert against clinical reference standard | 5c (869) | 22 (9.0–44); 95 (92–98) | 100 (66–100); 78 (59–97) | Not applicable | Not applicable | Not applicable |
| Stool Xpert against microbiological reference standard for children with HIV | 5d (395) | 79 (68–87); 0 (0–100) | 99 (94–100); 35 (0–99) | 5g (379) | 80 (68–88); 0 (0–100) | 99 (94–100); 51 (0–100) |
| Stool Xpert against microbiological reference standard for HIV-negative children | 7e (974) | 61 (40–79); 39 (0–100) | 99 (98–100); 56 (13–100) | Not applicable | Not applicable | Not applicable |
Results of the sensitivity analysis in which we excluded the study that did not use mycobacterial culture as part of the reference standard (15) are presented in Fig. S2. Pooled sensitivity and specificity estimates combining data from all studies and data stratified by HIV status were all similar to those estimated in our main analyses, as was between-study heterogeneity. Pooled estimates from our main analysis and from this sensitivity analysis are summarized in Table 3.
We undertook two post hoc sensitivity analyses. In the first, we sought to determine whether the quantity of stool used for testing was associated with diagnostic accuracy (assuming that a higher mass might increase sensitivity). There were too few studies to estimate pooled accuracy stratified by stool mass used; however, visual inspection of forest plots showed no obvious trend to support a minimum quantity (Fig. S3). In the second sensitivity analysis, we evaluated whether the burden of TB in the country where a study was conducted was associated with the accuracy of stool Xpert. As shown in Fig. S4, there was no clear trend to suggest such an association.
DISCUSSION
In this systematic review and meta-analysis, we found that the sensitivity and specificity of stool Xpert (67% [95% CI, 52 to 79%] and 99% [95% CI, 98 to 99%], respectively) for the diagnosis of microbiologically confirmed childhood PTB were comparable to what has been reported for the performance of Xpert on respiratory specimens (62% [95% credible interval, 51 to 73%] and 98% [95% credible interval, 97 to 99%], respectively) (4). Sensitivity and specificity varied by HIV status. As stool collection is noninvasive, this is of substantial interest for the medical evaluation of children with suspected PTB, but a number of limitations of the existing evidence highlight the need for more research, and greater standardization of testing, before policy formulation.
Among the most important limitations of the evidence base is the lack of data on performance in the subpopulation of children for whom stool Xpert is of greatest potential clinical utility, those under the age of 5 years, and especially the subgroup under the age of 2 years. Only one study compared accuracy between age categories, and a cutoff of 10 years of age was used (17).
We observed substantial between-study heterogeneity in diagnostic accuracy, mostly for sensitivity. Different approaches to participant selection likely contributed to this, in particular the use of a case-control design (15) and nonconsecutive sampling (16, 21), which are at a higher risk of introducing bias into a study. Data also suggested that heterogeneity was partly explained by differences in the prevalence of HIV infection. The higher sensitivity of stool Xpert among children with HIV has also been observed for other specimen types in this population (4, 24), perhaps as a result of more severe TB disease in HIV-TB-coinfected children.
We found substantial variability in protocols for performing stool Xpert, with each study taking a unique approach. Differences were seen at all steps: (i) at stool collection, different methods of sampling, numbers of specimens, and volumes of stool were used; (ii) different reagents were added to stool samples before homogenization, and all studies utilized different additional reagents; and (iii) dissimilar filtration methods and decontamination steps were adopted. Future studies should ensure, at minimum, complete reporting of protocols for stool collection processing and testing. A standardized protocol would be of value, as would a standardized stool collection-and-processing kit.
Our systematic review and meta-analysis has a number of strengths. First, all included studies reported using a microbiological reference standard for comparison to stool Xpert, and 8 out of 9 studies used liquid or solid culture. While the imperfect nature of any reference standard for diagnosing pediatric TB means that the true number of affected children is always unknown, the accuracy of stool Xpert against microbiological confirmation is likely a closer estimation of its true accuracy than its performance compared to the clinical reference standard (as symptoms of PTB are nonspecific). Second, by systematically assessing each study’s sample preparation and processing techniques, we found substantial variability in methods of performing stool Xpert and were also able to identify obstacles to implementation. For example, most protocols required at least one centrifugation step, which is inauspicious in terms of translating this assay to a lower health care system level. Finally, we utilized a sensitive and validated search strategy that covered six languages.
The present work also has some limitations. First, data were insufficient, and there were too few studies for us to perform stratified or metaregression analyses to assess most demographic-related potential causes of observed heterogeneity. Hence, we suggest that in addition to HIV-stratified results, future studies of stool Xpert should also ensure that reporting is stratified by age, gender, and extent of radiographic disease. Second, while we identified wide variability in sampling and stool processing, we could not explore these as sources of heterogeneity or determine if any processing workflows were potentially superior. Third, we did not include one study concerning the performance of stool Xpert on samples from children (25) that was reported after our systematic search was completed and therefore was not included in our meta-analysis. However, including it in our pooled analyses did not significantly alter sensitivity or specificity estimates (see Fig. S5 in the supplemental material). Finally, our pooled estimates came from study populations with a high prevalence of TB; hence, it is possible that these estimates may not be generalizable to settings of lower TB burdens.
Given that these preliminary studies of stool Xpert suggest high specificity and moderate sensitivity, its potential role in the diagnostic pathway would be as a first-line rule-in test rather than as a triage test to rule out PTB. Studies assessing whether stool Xpert has value as an add-on test in combination with currently deployed assays will be useful, as will studies assessing the effect of repeat testing on sensitivity.
Conclusion.
Preliminary data suggest that the use of Xpert on stool specimens may be potentially useful as a rule-in test, but a standardized stool sample preparation protocol is lacking, and the accuracy of stool Xpert in children under 5 years old, the subgroup for whom the test could bring the most added value, remains largely unknown.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Samuel Schumacher for his early critical comments regarding study protocol development.
We attest that all authors of this research paper have directly participated in the planning, execution, or analysis of the study and have seen and approved the manuscript.
This work was supported by the Foundation for Innovative New Diagnostics (FIND), Geneva, Switzerland (grant number OCC2015-412). Faiz Ahmad Khan receives salary support from the Fonds de Récherche du Québec Santé.
Emily MacLean has no conflict. Giorgia Sulis has no conflict. Claudia M. Denkinger is employed by FIND. It works closely with the private and public sectors and receives funding from some of its industry partners. It has organizational firewalls to protect it against any undue influences in its work or the publication of its findings. All industry partnerships are subject to review by an independent scientific advisory committee or another independent review body, based on due diligence, TTPs, and public-sector requirements. It provides indirect support to industry to facilitate the development and use of products in these areas. FIND also supports the evaluation of prioritized assays and the early stages of implementation of WHO-approved (guidance and PQ) assays using donor grants. In order to carry out test validations and evaluations, it has product evaluation agreements with several private-sector companies for the diseases that FIND works on which strictly define its independence and neutrality vis-à-vis the companies whose products are evaluated and describe roles and responsibilities. James C. Johnston has no conflict. Madhukar Pai reports no commercial or financial conflicts. He serves on the Scientific Advisory Committee of FIND, Geneva, Switzerland, a nonprofit foundation that works on diagnostics. Faiz Ahmad Khan has no conflict.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.02057-18.
REFERENCES
- 1.World Health Organization. 2018. Global tuberculosis report 2018. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Lombard C, Enarson DA, Beyers N. 2006. A refined symptom-based approach to diagnose pulmonary tuberculosis in children. Pediatrics 118:e1350–e1359. doi: 10.1542/peds.2006-0519. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. 2013. Policy update. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 4.Detjen AK, DiNardo AR, Leyden J, Steingart KR, Menzies D, Schiller I, Dendukuri N, Mandalakas AM. 2015. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. Lancet Respir Med 3:451–461. doi: 10.1016/S2213-2600(15)00095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham SM, Cuevas LE, Jean-Philippe P, Browning R, Casenghi M, Detjen AK, Gnanashanmugam D, Hesseling AC, Kampmann B, Mandalakas A, Marais BJ, Schito M, Spiegel HM, Starke JR, Worrell C, Zar HJ. 2015. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis 61(Suppl 3):S179–S187. doi: 10.1093/cid/civ581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordova J, Shiloh R, Gilman RH, Sheen P, Martin L, Arenas F, Caviedes L, Kawai V, Soto G, Williams DL, Zimic M, Escombe AR, Evans CA. 2010. Evaluation of molecular tools for detection and drug susceptibility testing of Mycobacterium tuberculosis in stool specimens from patients with pulmonary tuberculosis. J Clin Microbiol 48:1820–1826. doi: 10.1128/JCM.01161-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donald PR, Schaaf HS, Gie RP, Beyers N, Sirgel FA, Venter A. 1996. Stool microscopy and culture to assist the diagnosis of pulmonary tuberculosis in childhood. J Trop Pediatr 42:311–312. doi: 10.1093/tropej/42.5.311. [DOI] [PubMed] [Google Scholar]
- 8.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. 2011. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 9.Macaskill P, Gatsonis C, Deeks J, Harbord R, Takwoingi Y. 2010. Chapter 10: Analysing and presenting results In Deeks JJ, Bossuyt PM, Gatsonis C (ed), Cochrane handbook for systematic reviews of diagnostic test accuracy, version 1.0. The Cochrane Collaboration, London, United Kingdom: https://methods.cochrane.org/sites/methods.cochrane.org.sdt/files/public/uploads/Chapter%2010%20-%20Version%201.0.pdf. [Google Scholar]
- 10.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. 2005. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 58:982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, Altman DG. 2003. Measuring inconsistency in meta-analyses. BMJ 327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dwamena B. 2007. MIDAS: Stata module for meta-analytical integration of diagnostic test accuracy studies. Statistical software components S456880 Boston College Department of Economics, Boston, MA. [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 14.Orikiriza P, Nansumba M, Nyehangane D, Bastard M, Mugisha IT, Nansera D, Mwanga-Amumpaire J, Boum Y II, Kumbakumba E, Bonnet M. 2018. Xpert MTB/RIF diagnosis of childhood tuberculosis from sputum and stool samples in a high TB-HIV-prevalent setting. Eur J Clin Microbiol Infect Dis 37:1465–1473. doi: 10.1007/s10096-018-3272-0. [DOI] [PubMed] [Google Scholar]
- 15.Banada PP, Naidoo U, Deshpande S, Karim F, Flynn JL, O’Malley M, Jones M, Nanassy O, Jeena P, Alland D. 2016. A novel sample processing method for rapid detection of tuberculosis in the stool of pediatric patients using the Xpert MTB/RIF assay. PLoS One 11:e0151980. doi: 10.1371/journal.pone.0151980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasan Z, Shakoor S, Arif F, Mehnaz A, Akber A, Haider M, Kanji A, Hasan R. 2017. Evaluation of Xpert MTB/RIF testing for rapid diagnosis of childhood pulmonary tuberculosis in children by Xpert MTB/RIF testing of stool samples in a low resource setting. BMC Res Notes 10:473. doi: 10.1186/s13104-017-2806-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chipinduro M, Mateveke K, Makamure B, Ferrand RA, Gomo E. 2017. Stool Xpert MTB/RIF test for the diagnosis of childhood pulmonary tuberculosis at primary clinics in Zimbabwe. Int J Tuberc Lung Dis 21:161–166. doi: 10.5588/ijtld.16.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaCourse SM, Pavlinac PB, Cranmer LM, Njuguna IN, Mugo C, Gatimu J, Stern J, Walson JL, Maleche-Obimbo E, Oyugi J, Wamalwa D, John-Stewart G. 2018. Stool Xpert MTB/RIF and urine lipoarabinomannan for the diagnosis of tuberculosis in hospitalized HIV-infected children. AIDS 32:69–78. doi: 10.1097/QAD.0000000000001662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walters E, Van Der Zalm MM, Palmer M, Bosch C, Demers AM, Draper H, Goussard P, Schaaf HS, Friedrich SO, Whitelaw A, Warren R, Gie RP, Hesseling AC. 2017. Xpert MTB/RIF on stool is useful for the rapid diagnosis of tuberculosis in young children with severe pulmonary disease. Pediatr Infect Dis J 36:837–843. doi: 10.1097/INF.0000000000001563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcy O, Ung V, Goyet S, Borand L, Msellati P, Tejiokem M, Nguyen Thi NL, Nacro B, Cheng S, Eyangoh S, Pham TH, Ouedraogo AS, Tarantola A, Godreuil S, Blanche S, Delacourt C. 2016. Performance of Xpert MTB/RIF and alternative specimen collection methods for the diagnosis of tuberculosis in HIV-infected children. Clin Infect Dis 62:1161–1168. doi: 10.1093/cid/ciw036. [DOI] [PubMed] [Google Scholar]
- 21.Moussa H, Bayoumi FS, Mohamed AM. 2016. Gene Xpert for direct detection of Mycobacterium tuberculosis in stool specimens from children with presumptive pulmonary tuberculosis. Ann Clin Lab Sci 46:198–203. [PubMed] [Google Scholar]
- 22.Nicol MP, Spiers K, Workman L, Isaacs W, Munro J, Black F, Zemanay W, Zar HJ. 2013. Xpert MTB/RIF testing of stool samples for the diagnosis of pulmonary tuberculosis in children. Clin Infect Dis 57:e18–e21. doi: 10.1093/cid/cit230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. 2014. Xpert MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014:CD009593. doi: 10.1002/14651858.CD009593.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. 2016. The use of the Xpert MTB/RIF assay for the diagnosis of TB. Meeting report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 25.Walters E, Scott L, Nabeta P, Demers AM, Reubenson G, Bosch C, David A, van der Zalm M, Havumaki J, Palmer M, Hesseling AC, Ncayiyana J, Stevens W, Alland D, Denkinger C, Banada P. 2018. Molecular detection of Mycobacterium tuberculosis from stool in young children using a novel centrifugation-free processing method. J Clin Microbiol 56:e00781-18. doi: 10.1128/JCM.00781-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.