Abstract
Adult tissue stem cells have shown promise for the treatment of debilitating tendon injuries. However, few comparisons of stem cells from different tissue sources have been made to determine the optimum stem cell source for treating tendon. Moreover, it is likely that the application of tenogenic growth factors will improve tendon stem cell treatments further, and a comprehensive comparison of a number of growth factors is needed. Thus far, different types of stem cells cannot be evaluated in a high-throughput manner. To this end, we have developed an approach to culture mesenchymal stem cells isolated from bone marrow in collagen type I hydrogels with tenogenic growth factors using economical, commercially available supplies. To optimize growth factors for this assay, FGF-2, TGF-β1, IGF-1, and/or BMP-12 were tested singly and in novel combinations of (1) BMP-12 and IGF-1, (2) TGF-β1 and IGF-1, and/or (3) BMP-12 and FGF-2 over 10 days. Our data suggest that BMP-12 supplementation alone results in the strongest expression of tendon marker genes, controlled contractility of constructs, a higher degree of cell alignment, and tendon-like tissue morphology. This easy-to-use benchtop assay can be used to screen novel sources of stem cells and cell lines for tissue engineering and tendon healing applications.
Keywords: Tendon, growth factor, collagen hydrogel, mesenchymal stem cell
Introduction
Tendon injury is a significant clinical problem, and involved in over half the musculoskeletal injuries sustained each year.1 Human sporting activities frequently involve overuse or acute injuries of the Achilles tendon.2 Tendon injuries of the rotator cuff are prevalent in over 20% of the adult population, and incidence increases with age.3 The poor healing response in mature tendons results in a disorganized scar with inferior functional outcomes and a high incidence of reinjury.4 Mesenchymal stem cells (MSCs) have shown promise for the treatment of tendon injuries, with a reduced reinjury rate of treated superficial digital flexor tendons (SDFT) in horses5 and improved collagen organization and mechanical properties of Achilles tendons in humans.6 A major impediment to the development of MSC therapies for tendon healing is a lack of screening assays for optimal stem cells. Benchtop assays of tri-lineage differentiation, which are currently used to assess MSC potency, are poor indicators of tenogenesis.7 In the absence of a facile tenogenesis assay, novel sources of MSCs and different donor cell lines cannot be evaluated in an efficient and high-throughput manner.
Collagen type I comprises approximately 95% of the total collagen found in tendon.8 As a hydrogel, it is readily accessible and customizable in vitro. MSCs, by virtue of interacting actin and myosin filaments,9 can remodel an immobilized collagen hydrogel into a tendon-like form. Previous attempts to engineer tendon constructs in vitro have utilized silicone molds,10,11 synthetic sutures,12 vacuum pressure systems,13 and a variety of artificial biophysical agents. For example, Feng et al.11 designed rectangular silicone molds affixed with T-shaped silver wires at the mold edges for gel anchorage. Butler et al.12 customized glass petri dishes to incorporate small, rectangular troughs with tensioned synthetic sutures to aid gel attachment and contraction. Each of these systems relies on some or all of the following: careful manipulation and handling, expensive vessels or bioreactors that are impractical for high numbers of replicates, and custom manufacture of the apparatus.
Growth factor supplementation of MSCs facilitates tenogenesis, but there is incomplete understanding of which growth factors are sufficient and optimal. FGF-2 stimulates angiogenesis14 in vivo. TGF-β1 improves tendon biomechanical properties in rat patellar tendons.15 IGF-1 is an anti-inflammatory agent16 and promotes MSC chemotaxis at sites of injury14 in vivo. BMP-12 is primarily a tendon differentiation factor for MSCs,17 and BMP-12 tenogenesis has been previously reported via adenoviral gene transfer and protein supplementation in vitro,18,19 and BMP-12-releasing sutures in vivo.20 All of the aforementioned growth factors stimulate tendon-specific gene expression and matrix protein synthesis in vitro MSCs.21–23
While single tenogenic growth factors have been well investigated, their synergistic effects in tenogenesis are poorly understood. It is likely that growth factors work in concert during tendon repair and for the maintenance of tendon homeostasis in vivo. Combined effects of a few growth factors have been reported in vitro studies.24,25 For example, IGF-1 in concert with FGF-2 was shown to increase cell survival over single-factor supplemented scaffolds in one study.26 However, whether combined factors are truly more efficacious than single factors, and if yes, the optimal growth factor combination for tenogenesis is unknown. Further investigation is warranted to address these questions, and to understand the complex mechanisms of growth factor–mediated regeneration.
The goals of this study were to (1) develop a high-throughput, facile tenogenesis assay using commercially available materials and reagents and (2) determine from known tenogenic factors, which growth factors are sufficient and/or optimal for the tenogenic induction of bone marrow MSCs in our three-dimensional (3D) assay. The aforementioned growth factors (FGF-2, TGF-β1, IGF-1, and BMP-12) were evaluated individually, and in combination, and compared to unsupplemented 3D controls. BMP-12 was combined with either FGF-2 or IGF-1. In addition, TGF-β1 combined with IGF-1 was also evaluated as a third dual factor group.27 Tenogenesis was evaluated by collagen gel contraction, cell morphology and longitudinal alignment, gene expression of tendon markers, and glycosaminoglycan (GAG) content on day 10 of culture. We hypothesized that 3D culture of MSCs with BMP-12 and IGF-1, maintained under contraction-induced uniaxial strain, would result in a facile and practical tenogenesis assay. We further hypothesized that synergistic growth factors would augment stress-induced tenogenesis over individual factors.
Materials and methods
Experimental design
Previously isolated and cryopreserved bone marrow MSC lines derived from equine sternal bone marrow aspirate with Animal Care and Use Committee approval were used in this study (n = 3). Constructs were prepared by seeding collagen gel with bone marrow MSCs suspended in tenogenic growth media on day 0. On day 1 of culture, constructs were supplemented with one of four single growth factor cocktails, namely, (1) FGF-2, (2) TGF-β1, (3) IGF-1, or (4) BMP-12, or combined growth factor cocktails, namely, (1) BMP-12 and IGF-1, (2) TGF-β1 and IGF-1, or (3) BMP-12 and FGF-2. Recombinant human growth factors were purchased from commercial vendors. FGF-2 and IGF-1 were from BioVision (San Francisco, CA), TGF-β1 was from R&D Systems (Minneapolis, MA), and BMP-12 was purchased from Sigma-Aldrich (St. Louis, MI). The concentrations of each growth factor in media are described in Table 1. Each growth factor concentration was selected based on results of previous studies inducing tenogenesis.19,26,28 Each test group was plated in triplicate (n = 3), for a total of nine independent replicates (three technical replicates per donor were averaged prior to statistical analysis). Each replicate was represented by a stand-alone gel that was generated in separate culture tubes and wells from every other gel. For all outcome measures, growth factor groups were compared to 3D, unsupplemented controls. In addition, gene expression data were computed using an equine juvenile tendon reference control.
Table 1.
Growth factor treatment groups and concentrations of each growth factor.
| Growth factor concentration | Treatment groups |
||||||
|---|---|---|---|---|---|---|---|
| FGF-2 | TGF-β1 | IGF-1 | BMP-12 | BMP-12 IGF-1 |
TGF-β1 IGF-1 |
BMP-12 FGF-2 |
|
| FGF-2 | 5 ng/ml | 5 ng/ml | |||||
| TGF-β1 | 5 ng/ml | 5 ng/ml | |||||
| IGF-1 | 10 ng/ml | 10 ng/ml | 10 ng/ml | ||||
| BMP-12 | 50 ng/ml | 50 ng/ml | 50 ng/ml | ||||
Tenogenesis apparatus
Commercially available 4-welled, non-adherent, Nunc rectangular dishes (12.8 × 8.6 cm; Thermo Scientific, Waltham, MA) were purchased for this study. Each well in the plate was affixed with two sterile cloning cylinders (0.8 × 0.8 cm, Corning Inc., Christiansburg, VA) set 3 cm apart from each other along the longitudinal midline of the well, held in place by sterile silicone.
MSC culture and derivation of 3D constructs
MSC lines were previously determined to be positive for the expression of CD90, CD105, Oct-4, and undergo tri-lineage differentiation. Cells were expanded in culture for one passage. Upon confluence, cells were trypsinized and 1-million cells per gel were suspended in 5 mL of tenogenic growth media comprising high glucose DMEM (Thermo Scientific), 10% CellectTM Silver fetal bovine serum (MP Biomedicals, Santa Ana, CA), 37.5 μg/ml L-ascorbic acid (Sigma-Aldrich), 1% penicillin G (Sigma-Aldrich), and 0.8 mg/mL rat tail collagen I (Corning Life Sciences, Tewksbury, MA). Cell suspensions were plated immediately following preparation on day 0 and allowed to solidify in culture incubators maintained at 37°C, 5% CO2, and 90% humidity. After 1 hr of gelation, a sterile spatula was used to release the gels from the well walls to facilitate contraction. On day 1 of culture, test groups were supplemented with their designated media. All constructs were fed on alternate days during a 10-day culture period.
Cell morphology
Longitudinal sections of each construct were stained with a commercial two-color fluorescent assay (LIVE/DEAD Viability Kit; Thermo Scientific) for qualitative analysis. Construct images are representative of the average of six replicates and were acquired using a fluorescence microscope (EVOSTM FL Imaging System; Thermo Scientific). Independent samples were fixed in 4% paraformaldehyde overnight at 4°C, washed in PBS (phosphate-buffered saline), and submitted for histology (Laudier Histology, New York, NY). Samples were embedded in an acrylic resin, sectioned into 6-micron thick longitudinal slices and stained with Masson’s trichrome stain. Images were acquired using a phase-contrast microscope (Olympus Corp, Center Valley, PA).
Quantification of cell alignment
Two histological sections per representative sample were used to quantify cell alignment using built-in ImageJ software analytical tools.29 Fifty cellular angles per section were measured relative to the construct longitudinal axis. Parallel alignment to the construct longitudinal axis was assigned 0º, and angles of each cell relative to 0º (0º–90º) were used to construct histograms. The resulting histograms for each sample were averaged to draw group-wise comparisons.
Gel contraction analysis
On days 0, 1, 3, 5, 7, and 10, digital images of all constructs were taken to assess percentage area and determine contraction of the constructs. Images were analyzed using built-in ImageJ software analytical tools. The contracted area at each time point was calculated as a percentage of the initial area of the gel (% contraction).
Gene expression analysis
A section of each construct was used for gene expression analysis. Samples were homogenized in TRIzol reagent (Thermo Scientific) for RNA isolation according to the manufacturer’s protocol. Genomic DNA contamination of RNA pellets was removed using RNeasy spin columns and on-column DNase treatment (QIAGEN Inc., Germantown, MD), and purified RNA was quantified using a Nanodrop spectrophotometer. First-strand complimentary DNA (cDNA) synthesis was performed using a high-capacity reverse transcriptase kit (High-Capacity RNA-to-cDNA kit; Thermo Scientific). Real-time quantitative PCR (7500 Real-Time PCR System; Thermo Scientific) was performed using custom TaqMan-MGB probes and sequence detection primers (Thermo Scientific) designed with Primer ExpressTM software (Version 3.0; Thermo Scientific). The comparative threshold cycle method (2-ΔΔCt) was employed for relative quantification of gene expression.30 Data were normalized to GAPDH, which was validated for stability in our study using qbase+ software (Biogazelle, Zwijnaarde, Belgium). Tenogenic marker expression is reported as fold change with respect to a tendon reference control. A list of primers and probes is included in Table 2.
Table 2.
Custom-designed equine primer and probe sequences.
| Forward primer | Reverse primer | Probe | |
|---|---|---|---|
| GAPDH | CAAGTTCCATGGCACAGTCAAG | GGCCTTTCCGTTGATGACAA | CCGAGCACGGGAAG |
| Scleraxis | CGCCCAGCCCAAACAG | TTGCTCAACTTTCTCTGGTTGCT | TCTGCACCTTCTGCC |
| Collagen I | GCCAAGAAGAAGGCCAAGAA | TGAGGCCGTCCTGTATGC | ACATCCCAGCAGTCACCT |
| Collagen III | CTGCTTCATCCCACTCTTAT | ATCCGCATAGGACTGACCA | AACAGGAAGTTGCTGAAGG |
| Decorin | AAGTTGATGCAGCTAGCCTG | GGCCAGAGAGCCATTGTCA | ATTTGGCTAAATTGGGACTG |
| Biglycan | TGGACCTGCAGAACAATGAGAT | AGAGATGCTGGAGGCCTTTG | TCTGAGCTCCGAAAGG |
GAG quantification
Representative constructs from each replicate were homogenized overnight in a papain-containing digest buffer (Sigma-Aldrich) at 60°C. Cumulative GAG content within the construct was quantified by the 1,9 dimethylmethylene blue dye (Sigma-Aldrich) colorimetric assay,31 using chondroitin sulfate (Sigma-Aldrich) as a reference standard. Data were normalized to total DNA content as determined by a Nanodrop spectrophotometer. Data are reported as μg GAG/μg DNA.
Statistical analysis
Data were tested for normality using the Shapiro–Wilk test. Non-normal data were log transformed and achieved normality prior to analysis. For gel contraction, a one-way multivariate analysis of variance with a repeated-measures design and Student’s t-tests were used to assess differences between groups over time and at each time point. For gene expression and GAG data, a one-way analysis of variance was performed followed by post hoc Tukey’s tests for pairwise comparisons between groups. Cell alignment was assessed using Student’s t-tests. Quantitative data are reported as mean ± standard error. Distinct letters denote significant differences on graphical data for cell alignment, gene expression, and GAG content. For gel contraction, detailed statistical data are presented as supplemental tables. A p value of less than 0.05 was considered significant. Computation was performed in JMP Pro 15 (SAS Institute, Cary, NC) and MS Excel 11 (Microsoft, Redmond, WA).
Results
Growth factors modulate cell alignment and anisotropic contraction
In all groups, the MSCs uniformly integrated into the 3D gel constructs and progressively aligned with the longitudinal axis of tension (Figure 1). In all groups, >99% of MSCs exhibited elongated cell morphology and were viable. On day 10, MSCs in BMP-12 constructs were significantly better aligned when compared with control, FGF-2, BMP-12/FGF-2, and TGF-β1/IGF-1 constructs (Figure 2).
Figure 1.
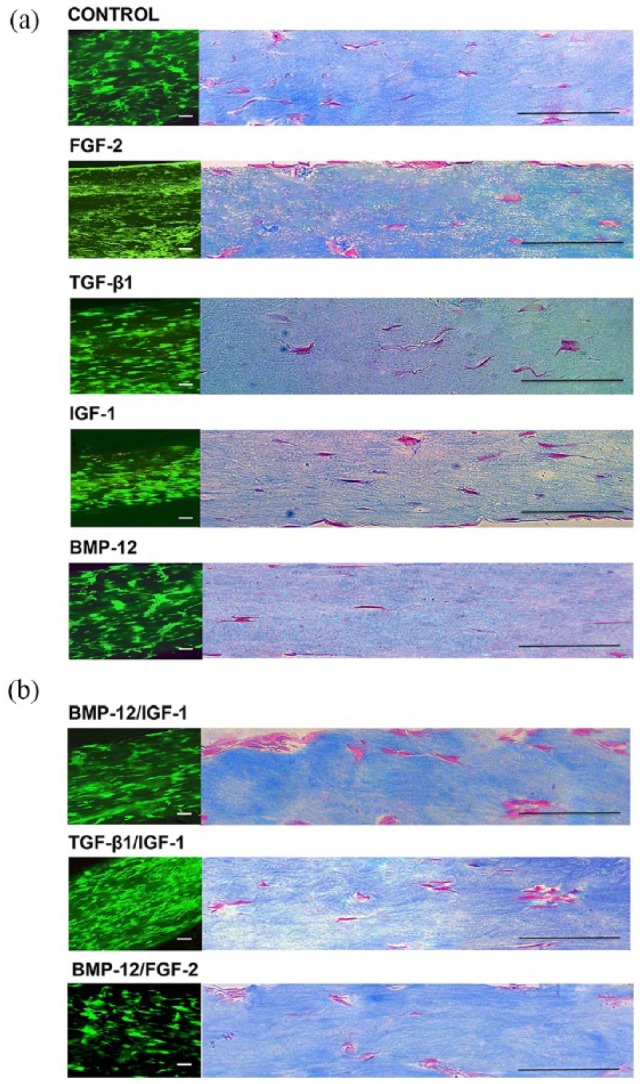
Fluorescence microscopy (a) and Masson’s trichrome histology images (b) of day-10 gels. All constructs exhibited uniform integration of MSCs in three dimensions. MSCs were highly aligned to the axis of tension. Scale bars represent 125 μm.
Figure 2.
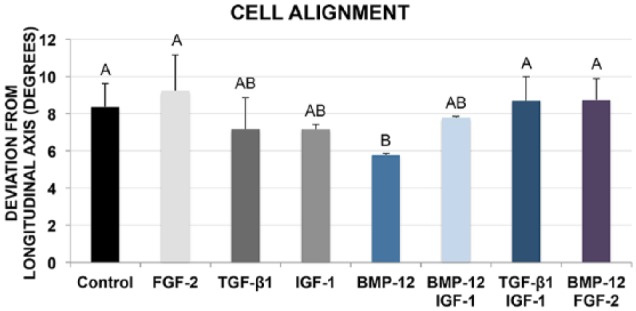
Angle of deviation from the longitudinal construct axis for day-10 constructs, with perfect longitudinal alignment = 0º. BMP-12 enhanced parallel cell alignment significantly more than control, FGF-2, TGF-β1/IGF-1, and BMP-12/FGF–2. Data points that do not share a letter are significantly different.
Gel contraction (contracted gel area) was significantly improved in each group over time, and significant differences were seen in between groups at each time point. From day 0 to day 3, all groups contracted rapidly to 20%–50% of the initial area, whereupon the rate of contraction decreased (Figure 3(b)). The BMP-12 group was significantly more contracted than TGF-β1 and BMP-12/FGF-2 on days 5, 7, and 10. No significant differences were noted between BMP-12 and BMP-12/IGF-1. TGF-β1/IGF-1 contracted the most of any group and significantly more than TGF-β1 and BMP-12/FGF-2 at most time points (days 3, 5, 7, and 10). BMP-12/FGF-2 contracted the least of any group and was significantly less contracted than the control, FGF-2, BMP-12, BMP-12/IGF-1, and TGF-β1/IGF-1 groups at most time points (days 3, 5, 7, and 10). The remainder of the results is presented in Supplemental Tables 1–5.
Figure 3.
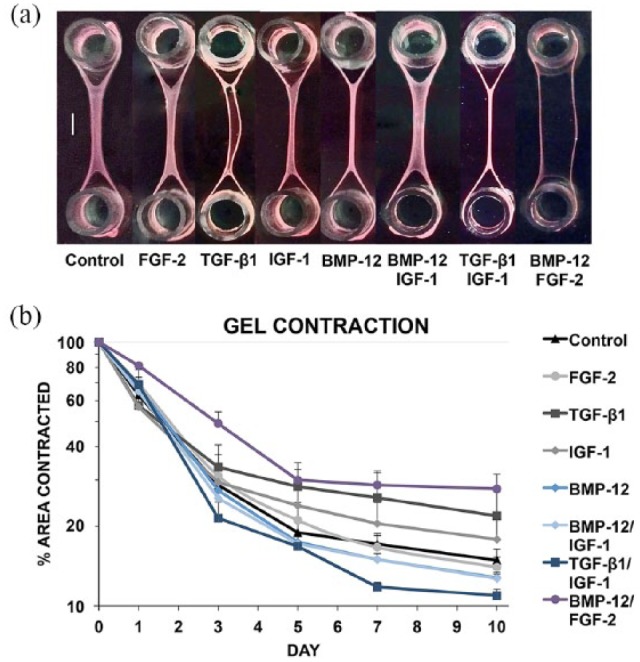
Digital images of constructs at harvest (a) and graphical representation in logarithmic scale of percentage contraction of constructs over 10 days of culture (b). Within group, comparisons showed that each group had contracted gel area from the previous time point. BMP12/FGF2 constructs had contracted the least at culture endpoint, and TGF-β1/IGF-1 constructs had contracted the most. Scale bar on images represents 5000 µm.
Tendon gene expression in 3D culture is augmented by BMP-12
Control constructs expressed all tendon-related genes evaluated in this study on day 10, and the addition of BMP-12 and BMP-12/IGF-1 augmented gene expression toward a tendon phenotype (Figure 4). BMP-12 induced more than a threefold increase of scleraxis over controls (p = 0.0338). BMP-12-mediated matrix remodeling was evident by more than a fivefold increase of collagen type III expression over TGF-β1 (p = 0.0116) and TGF-β1/IGF-1 (p = 0.0172). BMP-12/IGF-1 significantly increased collagen type III expression over all groups except BMP-12, BMP-12/FGF-2, and control. Decorin expression was the greatest in BMP-12 and BMP-12/IGF-1, significantly greater than the control, FGF-2, TGF-β1, and TGF-β1/IGF-1 groups. Biglycan expression remained unchanged in response to growth factor treatment. Interestingly, combining FGF-2 with BMP-12 significantly decreased BMP-12-induced scleraxis expression (p = 0.0215). Consistent with this, BMP-12/FGF-2 expression of collagen type I was significantly lower than IGF-1 (p = 0.0186), although the level was not significantly reduced from other groups.
Figure 4.
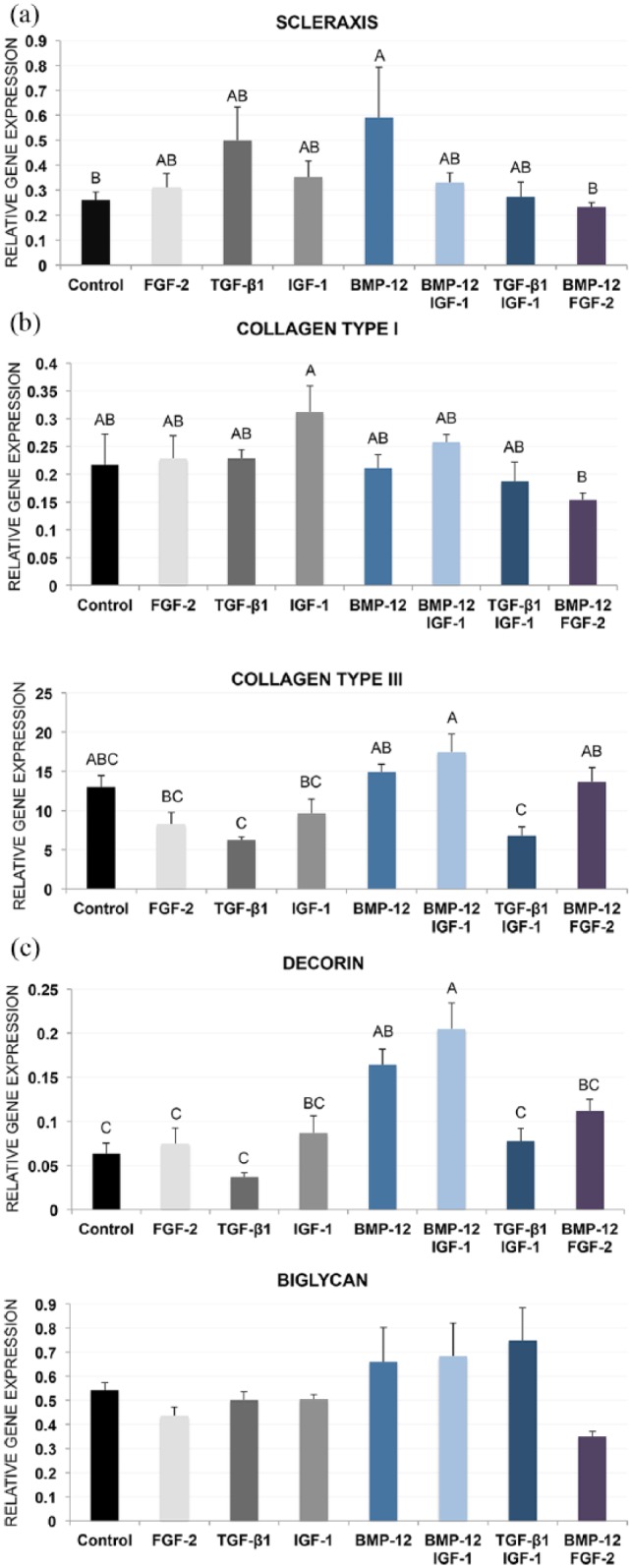
Gene expression profiles of tendon marker genes scleraxis (a), collagen types I and III (b), and decorin and biglycan (c). BMP-12 and BMP-12/IGF-1 constructs consistently increased expression of all markers on day 10. Data are reported as fold change with respect to a tendon reference control. Data points that do not share a letter are significantly different.
Growth factors in combination increase cumulative GAG content
Single growth factors in this study failed to increase cumulative GAG content within the constructs compared to control (Figure 5). BMP-12 combined with IGF-1, resulted in a twofold increase in GAG composition over control (p = 0.0427) and all single growth factor groups. TGF-β1/IGF-1 and BMP-12/FGF-2 also increased GAG compared with control and single growth factor groups. Interestingly, FGF-2 had significantly lower GAG compared with control (p = 0.0483). No other significant differences were detected.
Figure 5.
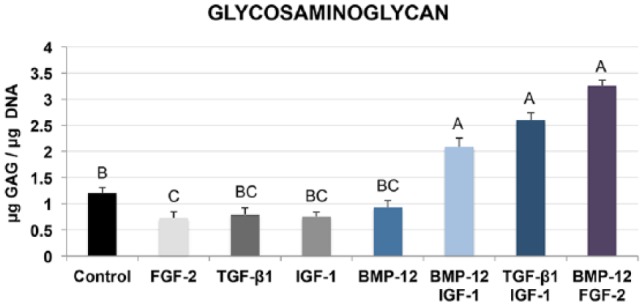
Glycosaminoglycan levels of day-10 constructs were assessed using the 1,9 dimethylmethylene blue dye assay. Synergistic effects of dual factor groups were detected for GAG. BMP-12 combined with IGF-1, synergistically increased GAG levels. Endpoint GAG content in the BMP-12/FGF-2 constructs was the highest at harvest, significantly greater than all single factor groups and control. Data points that do not share a letter are significantly different.
Discussion
The overarching goal of this study was to develop an easy-to-use tenogenesis assay for the efficient and relatively rapid evaluation of candidate stem cells for tendon engineering and treatment of tendon injuries. Toward this end, we designed and validated a simple apparatus using commercially available plasticware for the application of uniaxial static strain. We determined that BMP-12 protein supplementation of bone marrow–derived stem cells in this tenogenesis apparatus induces a composite tendon phenotype over 10 days.
Native tendon tissue is characterized by a highly organized array of cells and collagen fibrils, aligned in parallel to the longitudinal axis of tension.32 Progressive cellular alignment in response to tissue anisotropy is a hallmark of tenogenesis,33 and was observed in all constructs over the culture period. Furthermore, to the best of our knowledge, this study is the first to perform quantitative comparisons of MSC orientation in response to exogenous growth factors during differentiation. The increased cell alignment in response to BMP-12 in this study further strengthens the hypothesis that BMP-12 augments strain-mediated tendon tissue morphology in vitro34; however, this effect was reduced by the BMP-12/FGF-2 group.
BMP-12 and BMP-12/IGF-1 stimulated the greatest overall increases in tendon gene expression compared to other growth factor groups. The increased expression of scleraxis (a bHLH transcription factor)35 in response to BMP-12 was expected, since BMP-12 is well documented in the literature as a tenogenic growth factor.25,36 BMP-12 has been shown to augment strain-induced scleraxis expression in MSCs, establishing a combined effect of uniaxial strain and BMP-12,37 which was also observed in this study. Decorin and biglycan are small leucine-rich proteoglycans (SLRP) abundantly found in the tendon matrix.38 Expression of decorin in response to BMP-12 in MSCs has previously reported contradicting results; some studies report significant increases,39 while others report unaffected expression.40 In this study, the strong upregulation of decorin expression in BMP-12 and BMP-12/IGF-1 constructs may explain the lack of induction of biglycan observed in the same groups. Decorin and biglycan may functionally compensate for each other, evident by the increasing expression of biglycan in response to loss of decorin in vivo.41
The in vitro process of collagen gel contraction by tenocytes recapitulates their physiological behavior to remodel a collagenous matrix in response to an external stimulus, such as strain42 or inflammation.43 A ratio of 1-million cells to 4-mg collagen in this study resulted in a reduction of collagen gel area to 29% of the initial area by day 3, and to 18% by day 7, consistent with previous results.44 TGF-β1 has been shown to enhance the contraction of tenocyte-seeded matrices under strain.10 In this study, TGF-β1 independently did not increase contraction over controls; however, the synergistic effects of TGF-β1 and IGF-1 augmented tissue contractility over both individual factors. Wound healing in rabbit patellar tendons is enhanced with the combined application of TGF-β1 and IGF-1,27 which may be attributed to enhanced tenocyte contraction and GAG synthesis. BMP-12 (singly and combined with IGF-1) construct contraction was not significantly different from TGF-β1/IGF-1, in support of its role as a tendon healing agent in vivo.45 When FGF-2 was combined with BMP-12, contraction was significantly reduced compared to either factor alone.
GAGs are the side chains of tendon proteoglycans.46 The cumulative GAG content in control constructs (1.2 μg GAG/μg DNA) is consistent with the physiologic levels of equine SDFT (0.2–0.8 μg GAG/μg DNA).47 An increased accumulation of GAGs, resulting from a synergistic effect of BMP-12 and IGF-1 in this study, is expected during tendon neogenesis48 (2.1 μg GAG/μg DNA compared to 1.2 μg GAG/μg DNA of control constructs). Furthermore, this observation may be correlated to a similar increase in decorin expression in the same dual factor group. BMP-12 independently did not influence GAG levels, contrary to one previous report.49
Results from this study support the claim that BMP-12 is primarily a tendon differentiation factor, whereas FGF-2, TGF-β1, and IGF-1 may be better described as inducers of matrix synthesis and/or cell proliferation.14,50,51 However, our study is not without limitations. Bioactive levels of growth factors were selected in this study,17,19,22,24,26,28 and a single concentration of each growth factor was evaluated. Furthermore, outcomes from this study represent a single time point in the culture period. It may be beneficial to test outcomes from different concentrations of growth factors at several time points using this assay, to understand their spatiotemporal control of tenogenesis.
Our investigation of synergistic growth factors in this study is comprehensive and accurate. In this study, (1) all single growth factor groups were incorporated and (2) single factor groups were incorporated in parallel rather than separate experiments with combined factor groups, so that accurate assessments of growth factor synergism could be made. In addition to the synergistic effects described above and in this study, an inhibitory effect of FGF-2 on BMP-12 was observed, which has not been previously reported. Specifically, BMP-12-induced MSC alignment, contraction and induction of scleraxis gene expression were inhibited with the addition of FGF-2. Adenoviral transfer of BMP-12 did not affect the endogenous production of FGF-2 in a previous study of rat Achilles tendons.52 In contrast, results from this study suggest that the simultaneous overexpression of FGF-2 and BMP-12 may downregulate tenogenic differentiation, further suggesting cross talk in these two growth factors. Addition of IGF-1 to BMP-12 constructs did not augment tenogenesis over BMP-12 alone, except for an increase in GAG content. Hence, for the purpose of this assay, BMP-12 is sufficient to induce tenogenic differentiation. The effects of BMP-12 were manifested by the stronger expression of tenogenic genes, controlled contractility of constructs, a substantial degree of cell alignment, and tendon-like tissue morphology.
Conclusion
This novel benchtop assay does not require sophisticated materials or machinery. Uniaxial static tension combined with BMP-12 is sufficient to induce tenogenic differentiation within a 10-day culture period. This assay can be used to assess large numbers of donor MSC cell lines for the optimum stem cells for allogeneic treatments. This would enable off-the-shelf treatment of tendon injury.
Supplemental Material
Supplemental material, Supplemental_Table_1_(1) for Optimizing growth factor induction of tenogenesis in three-dimensional culture of mesenchymal stem cells by Ibtesam Rajpar and Jennifer G Barrett in Journal of Tissue Engineering
Supplemental Material
Supplemental material, Supplemental_Table_2 for Optimizing growth factor induction of tenogenesis in three-dimensional culture of mesenchymal stem cells by Ibtesam Rajpar and Jennifer G Barrett in Journal of Tissue Engineering
Supplemental Material
Supplemental material, Supplemental_Table_3 for Optimizing growth factor induction of tenogenesis in three-dimensional culture of mesenchymal stem cells by Ibtesam Rajpar and Jennifer G Barrett in Journal of Tissue Engineering
Supplemental Material
Supplemental material, Supplemental_Table_4 for Optimizing growth factor induction of tenogenesis in three-dimensional culture of mesenchymal stem cells by Ibtesam Rajpar and Jennifer G Barrett in Journal of Tissue Engineering
Supplemental Material
Supplemental material, Supplemental_Table_5 for Optimizing growth factor induction of tenogenesis in three-dimensional culture of mesenchymal stem cells by Ibtesam Rajpar and Jennifer G Barrett in Journal of Tissue Engineering
Supplemental Material
Supplemental material, Supplementary_Fig_1 for Optimizing growth factor induction of tenogenesis in three-dimensional culture of mesenchymal stem cells by Ibtesam Rajpar and Jennifer G Barrett in Journal of Tissue Engineering
Acknowledgments
The authors would like to acknowledge Abdulkareem Zenhom for his contributions to this study, and the Aspiring Scientists Summer Internship Program at George Mason University.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The author(s) received financial support for this research through Dr. Barrett’s Theodora Ayer Randolph Professorship, and publication fees through the Virginia Tech Open Access Subvention Fund.
ORCID iD: Ibtesam Rajpar  https://orcid.org/0000-0003-1767-9921
https://orcid.org/0000-0003-1767-9921
Supplemental material: Supplemental material for this article is available online.
References
- 1. Thomopoulos S, Parks WC, Rifkin DB, et al. Mechanisms of tendon injury and repair. J Orthop Res 2015; 33: 832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jarvinen TA, Kannus P, Paavola M, et al. Achilles tendon injuries. Curr Opin Rheumatol 2001; 13: 150–155. [DOI] [PubMed] [Google Scholar]
- 3. Itoi E. Rotator cuff tear: physical examination and conservative treatment. J Orthop Sci 2013; 18(2): 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith RK, Werling NJ, Dakin SG, et al. Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS ONE 2013; 8(9): e75697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Godwin EE, Young NJ, Dudhia J, et al. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J 2012; 44(1): 25–32. [DOI] [PubMed] [Google Scholar]
- 6. Chong AK, Ang AD, Goh JC, et al. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit Achilles tendon model. J Bone Joint Surg Am 2007; 89(1): 74–81. [DOI] [PubMed] [Google Scholar]
- 7. Gulotta LV, Chaudhury S, Wiznia D. Stem cells for augmenting tendon repair. Stem Cells Int 2012; 2012: 291431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riley GP, Harrall RL, Constant CR, et al. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis 1994; 53(6): 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takayama Y, Mizumachi K. Effects of lactoferrin on collagen gel contractile activity and myosin light chain phosphorylation in human fibroblasts. FEBS Lett 2001; 508(1): 111–116. [DOI] [PubMed] [Google Scholar]
- 10. Farhat YM, Al-Maliki AA, Chen T, et al. Gene expression analysis of the pleiotropic effects of TGF-beta1 in an in vitro model of flexor tendon healing. PLoS ONE 2012; 7(12): e51411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feng Z, Tateishi Y, Nomura Y, et al. Construction of fibroblast-collagen gels with orientated fibrils induced by static or dynamic stress: toward the fabrication of small tendon grafts. J Artif Organs 2006; 9(4): 220–225. [DOI] [PubMed] [Google Scholar]
- 12. Butler DL, Juncosa-Melvin N, Boivin GP, et al. Functional tissue engineering for tendon repair: a multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res 2008; 26(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 13. Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A 2008; 14(10): 1615–1627. [DOI] [PubMed] [Google Scholar]
- 14. Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med 2003; 33(5): 381–394. [DOI] [PubMed] [Google Scholar]
- 15. Yin Z, Guo J, Wu TY, et al. Stepwise differentiation of mesenchymal stem cells augments tendon-like tissue formation and defect repair in vivo. Stem Cells Transl Med 2016; 5(8): 1106–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kurtz CA, Loebig TG, Anderson DD, et al. Insulin-like growth factor I accelerates functional recovery from Achilles tendon injury in a rat model. Am J Sports Med 1999; 27(3): 363–369. [DOI] [PubMed] [Google Scholar]
- 17. Liu J, Tao X, Chen L, et al. CTGF positively regulates BMP12 induced tenogenic differentiation of tendon stem cells and signaling. Cell Physiol Biochem 2015; 35(5): 1831–1845. [DOI] [PubMed] [Google Scholar]
- 18. Lou J, Tu Y, Ludwig FJ, et al. Effect of bone morphogenetic protein-12 gene transfer on mesenchymal progenitor cells. Clin Orthop Relat Res 1999; 369: 333–339. [DOI] [PubMed] [Google Scholar]
- 19. Violini S, Ramelli P, Pisani LF, et al. Horse bone marrow mesenchymal stem cells express embryo stem cell markers and show the ability for tenogenic differentiation by in vitro exposure to BMP-12. BMC Cell Biol 2009; 10: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chamberlain CS, Lee JS, Leiferman EM, et al. Effects of BMP-12-releasing sutures on Achilles tendon healing. Tissue Eng Part A 2015; 21(5–6): 916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reed SA, Johnson SE. Expression of scleraxis and tenascin C in equine adipose and umbilical cord blood derived stem cells is dependent upon substrata and FGF supplementation. Cytotechnology 2014; 66(1): 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holladay C, Abbah SA, O’Dowd C, et al. Preferential tendon stem cell response to growth factor supplementation. J Tissue Eng Regen Med 2014; 10(9):783–798. [DOI] [PubMed] [Google Scholar]
- 23. Herchenhan A, Bayer ML, Eliasson P, et al. Insulin-like growth factor I enhances collagen synthesis in engineered human tendon tissue. Growth Horm IGF Res 2015; 25(1): 13–19. [DOI] [PubMed] [Google Scholar]
- 24. Caliari SR, Harley BA. Composite growth factor supplementation strategies to enhance tenocyte bioactivity in aligned collagen-GAG scaffolds. Tissue Eng Part A 2013; 19(9–10): 1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bottagisio M, Lopa S, Granata V, et al. Different combinations of growth factors for the tenogenic differentiation of bone marrow mesenchymal stem cells in monolayer culture and in fibrin-based three-dimensional constructs. Differentiation 2017; 95: 44–53. [DOI] [PubMed] [Google Scholar]
- 26. Farnebo S, Farnebo L, Kim M, et al. Optimized repopulation of tendon hydrogel: synergistic effects of growth factor combinations and adipose-derived stem cells. Hand (N Y) 2017; 12(1): 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lyras DN, Kazakos K, Verettas D, et al. Effect of combined administration of transforming growth factor-b1 and insulin-like growth factor I on the mechanical properties of a patellar tendon defect model in rabbits. Acta Orthop Belg 2010; 76(3): 380–386. [PubMed] [Google Scholar]
- 28. Klein MB, Yalamanchi N, Pham H, et al. Flexor tendon healing in vitro: effects of TGF-beta on tendon cell collagen production. J Hand Surg Am 2002; 27(4): 615–620. [DOI] [PubMed] [Google Scholar]
- 29. Liu ZQ. Scale space approach to directional analysis of images. Appl Opt 1991; 30(11): 1369–1373. [DOI] [PubMed] [Google Scholar]
- 30. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25(4): 402–408. [DOI] [PubMed] [Google Scholar]
- 31. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 1986; 883(2): 173–177. [DOI] [PubMed] [Google Scholar]
- 32. Maffulli N, Barrass V, Ewen SW. Light microscopic histology of achilles tendon ruptures. Am J Sports Med 2000; 28(6): 857–863. [DOI] [PubMed] [Google Scholar]
- 33. Foolen J, Wunderli SL, Loerakker S, et al. Tissue alignment enhances remodeling potential of tendon—derived cells—Lessons from a novel microtissue model of tendon scarring. Matrix Biol 2018; 65: 14–29. [DOI] [PubMed] [Google Scholar]
- 34. Lee JY, Zhou Z, Taub PJ, et al. BMP-12 treatment of adult mesenchymal stem cells in vitro augments tendon-like tissue formation and defect repair in vivo. PLoS ONE 2011; 6(3): e17531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schweitzer R, Chyung JH, Murtaugh LC, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 2001; 128(19): 3855–3866. [DOI] [PubMed] [Google Scholar]
- 36. Shen H, Gelberman RH, Silva MJ, et al. BMP12 induces tenogenic differentiation of adipose-derived stromal cells. PLoS ONE 2013; 8(10): e77613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li Y, Ramcharan M, Zhou Z, et al. The role of scleraxis in fate determination of mesenchymal stem cells for tenocyte differentiation. Sci Rep 2015; 5: 13149. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Dunkman AA, Buckley MR, Mienaltowski MJ, et al. The tendon injury response is influenced by decorin and biglycan. Ann Biomed Eng 2014; 42(3): 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stanco D, Vigano M, Perucca Orfei C, et al. Multidifferentiation potential of human mesenchymal stem cells from adipose tissue and hamstring tendons for musculoskeletal cell-based therapy. Regen Med 2015; 10(6): 729–743. [DOI] [PubMed] [Google Scholar]
- 40. Zarychta-Wisniewska W, Burdzinska A, Kulesza A, et al. BMP-12 activates tenogenic pathway in human adipose stem cells and affects their immunomodulatory and secretory properties. BMC Cell Biol 2017; 18(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang G, Ezura Y, Chervoneva I, et al. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem 2006; 98(6): 1436–1449. [DOI] [PubMed] [Google Scholar]
- 42. Shah JS, Palacios E, Palacios L. Development of crimp morphology and cellular changes in chick tendons. Dev Biol 1982; 94(2): 499–504. [DOI] [PubMed] [Google Scholar]
- 43. Galloway MT, Lalley AL, Shearn JT. The role of mechanical loading in tendon development, maintenance, injury, and repair. J Bone Joint Surg Am 2013; 95(17): 1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nirmalanandhan VS, Levy MS, Huth AJ, et al. Effects of cell seeding density and collagen concentration on contraction kinetics of mesenchymal stem cell-seeded collagen constructs. Tissue Eng 2006; 12(7): 1865–1872. [DOI] [PubMed] [Google Scholar]
- 45. Lou J, Tu Y, Burns M, et al. BMP-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res 2001; 19(6): 1199–1202. [DOI] [PubMed] [Google Scholar]
- 46. Rigozzi S, Muller R, Stemmer A, et al. Tendon glycosaminoglycan proteoglycan sidechains promote collagen fibril sliding-AFM observations at the nanoscale. J Biomech 2013; 46(4): 813–818. [DOI] [PubMed] [Google Scholar]
- 47. Hazeleger E. The biochemical differences of the superficial digital flexor tendon and the common digital extensor tendon between Warmbloods, Friesians and Thoroughbreds. Master’s Thesis, Department of Equine Sciences, Utrecht University, Utrecht, 2013. [Google Scholar]
- 48. Dahlgren LA, Mohammed HO, Nixon AJ. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J Orthop Res 2005; 23(1): 84–92. [DOI] [PubMed] [Google Scholar]
- 49. Mikic B, Bierwert L, Tsou D. Achilles tendon characterization in GDF-7 deficient mice. J Orthop Res 2006; 24(4): 831–841. [DOI] [PubMed] [Google Scholar]
- 50. Evans CH. Cytokines and the role they play in the healing of ligaments and tendons. Sports Med 1999; 28(2): 71–76. [DOI] [PubMed] [Google Scholar]
- 51. Haddad-Weber M, Prager P, Kunz M, et al. BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy 2010; 12(4): 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heisterbach PE, Todorov A, Fluckiger R, et al. Effect of BMP-12, TGF-beta1 and autologous conditioned serum on growth factor expression in Achilles tendon healing. Knee Surg Sports Traumatol Arthrosc 2012; 20(10): 1907–1914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Table_1_(1) for Optimizing growth factor induction of tenogenesis in three-dimensional culture of mesenchymal stem cells by Ibtesam Rajpar and Jennifer G Barrett in Journal of Tissue Engineering
Supplemental material, Supplemental_Table_2 for Optimizing growth factor induction of tenogenesis in three-dimensional culture of mesenchymal stem cells by Ibtesam Rajpar and Jennifer G Barrett in Journal of Tissue Engineering
Supplemental material, Supplemental_Table_3 for Optimizing growth factor induction of tenogenesis in three-dimensional culture of mesenchymal stem cells by Ibtesam Rajpar and Jennifer G Barrett in Journal of Tissue Engineering
Supplemental material, Supplemental_Table_4 for Optimizing growth factor induction of tenogenesis in three-dimensional culture of mesenchymal stem cells by Ibtesam Rajpar and Jennifer G Barrett in Journal of Tissue Engineering
Supplemental material, Supplemental_Table_5 for Optimizing growth factor induction of tenogenesis in three-dimensional culture of mesenchymal stem cells by Ibtesam Rajpar and Jennifer G Barrett in Journal of Tissue Engineering
Supplemental material, Supplementary_Fig_1 for Optimizing growth factor induction of tenogenesis in three-dimensional culture of mesenchymal stem cells by Ibtesam Rajpar and Jennifer G Barrett in Journal of Tissue Engineering


