Abstract
Objective:
Osteosarcoma is a common malignant bone tumor that is frequently found in the long bones of children and adolescents. The aim of this study is to examine long noncoding RNA-steroid receptor RNA activator 1 expression in osteosarcoma to explore the biological function of long noncoding RNA steroid receptor RNA activator 1 on proliferation, migration, and invasion along with apoptosis and its regulatory mechanism, which would facilitate the early diagnosis and targeted therapy of osteosarcoma.
Methods:
First, microarray analysis was applied to determine the expression of long noncoding RNAs in osteosarcoma tissues and paired normal tissues. Then, quantitative real-time polymerase chain reaction was utilized to validate microarray findings. Next, osteosarcoma cancerous cell lines SJSA-1 and U2OS were transfected with pcDNA3.1-SRA1 or pCMV-sh-SRA1 to increase or decrease steroid receptor RNA activator 1 expression levels, and microRNA-208a inhibitors, mimic to investigate the effects of microRNA-208a on osteosarcoma as well as the regulatory relation between long noncoding RNA steroid receptor RNA activator 1 and microRNA-208a. Cell proliferation was evaluated through Cell Counting Kit-8 and colony formation assays. Flow cytometry analysis was conducted to evaluate the apoptosis ratio. The migration and invasion abilities were measured using wound-healing and transwell assays.
Results:
Long noncoding RNA-steroid receptor RNA activator 1 expression was downregulated in osteosarcoma tissues and cells compared with that in corresponding normal tissues, whereas microRNA-208a expression was upregulated in osteosarcoma tissues. Moreover, the restoration of long noncoding RNA steroid receptor RNA activator 1 inhibited cell proliferation, and upregulation of long noncoding RNA steroid receptor RNA activator 1 restrained cell migration and invasion but boosted the apoptosis rate in osteosarcoma cells. In addition, long noncoding RNA steroid receptor RNA activator 1 targeting microRNA-208a was involved in the progression of osteosarcoma. Furthermore, upregulating microRNA-208a exerted similar roles of silencing long noncoding RNA steroid receptor RNA activator 1 in cell apoptosis, proliferation, migration, and invasion, which were reversed by enhancing the expression of long noncoding RNA steroid receptor RNA activator 1.
Conclusions:
In our study, long noncoding RNA steroid receptor RNA activator 1 played an antitumor role in osteosarcoma as it reduced cell migration, invasion, and proliferation, but facilitated cell apoptosis via sponging microRNA-208a, which could be regarded as a potential therapeutic target of osteosarcoma treatment.
Keywords: osteogenic sarcoma, long noncoding RNA, cell multiplication, programmed cell death, steroid receptor RNA activator 1
Introduction
Osteosarcoma (OS) is a common malignant bone tumor that is frequently found in the long bones of children and adolescents.1 Tumor cells can move through the cortex and medullary cavity and transfer through the blood to other tissues.2,3 Studies have shown that small amounts of tumor cells could be transferred to the brain, prostate, and other tissues, leading to death.4 Therefore, the prognosis of OS was dismal, and the 5-year survival rate was only 10% in patients with synchronous metastasis. Moreover, approximately 30% of patients would die from the disease without any evident metastasis. This frustrating condition was associated with the lack of extensive studies on this rare disease.5 Therefore, this study was designed to explore the regulatory function of steroid receptor RNA activator 1 (SRA1) on OS cell proliferation and apoptosis, hoping to improve the understanding of this disease.
Long noncoding RNAs (lncRNAs) are nonprotein-coding transcripts that can regulate a variety of physiological and pathological functions.6 Accumulating evidence shows that lncRNAs play pivotal roles in various biological processes of cancer cells, and their abnormal expression is closely related to cancer initiation and progression.7 In addition, lncRNAs can be used for post-transcriptional regulation, organization of protein complexes, cell-–cell signaling, and allosteric regulation of proteins and are considered as potential biomarkers for the prediction of cancers and tumor.8 In the field of OS research, a number of different lncRNAs have been identified to play critical roles, and relevant molecular mechanisms are complex accompanied by various genetic abnormal expressions. For instance, lncRNA HOXA distal transcript antisense RNA expression was amplified in patients with OS and was associated with poor prognosis9; hepatocellular carcinoma upregulated lncRNA increase contributed to OS cell metastasis,10 and lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) facilitated OS development.11 Recently, the SRA1, an lncRNA that functions as an RNA coactivator, has been suggested to play a vital role in myogenesis, steroidogenesis, breast tumorigenesis, and cardiomyopathy.12 It was found to be abnormally expressed in a series of human tumors. For example, the previous study demonstrated that SRA1 served as a cancer suppressor in hepatocellular carcinoma13 and was involved in breast cancer development.14 However, the association between SRA1 expression and tumorigenesis in OS remained unclear.
MicroRNAs (miRNAs) are one kind of short noncoding RNAs that could involve regulating gene expression by inducing mRNA cleavage or translational suppression and are highly conserved. Dysregulation of miRNAs was prevalent in various cancers, for example, renal cell carcinoma15 and non-small cell lung cancer.16 Meanwhile, lncRNAs usually sponge miRNA to exert function in various diseases. The miR-208a was upregulated in gastric cancer result in enhancing cell proliferation and invasion of gastric cancer.17 Yin et al indicated that miR-208a-3p suppressed cell apoptosis by targeting PDCD4 in gastric cancer.18
In our study, we aimed to examine lncRNA SRA1 and miR-208a expression in OS, to explore the biological function of lncRNA SRA1 on cell proliferation, migration, invasion, and apoptosis and its molecular regulatory mechanism in SJSA-1 and U2OS cell lines, which may facilitate the early diagnosis and target therapy of OS.
Materials and Methods
Patients and Tissues
Osteosarcoma tissues and their matched healthy tissues were acquired from 30 patients at Taizhou People’s Hospital. Freshly collected tissues were immediately frozen in liquid nitrogen. None of the patients received radiotherapy or chemotherapy before surgery. The use of the tissue samples was approved by the Ethics Committee of the Taizhou People’s Hospital. Written consent was obtained from all patients before they were included in the experiments.
Cell Culture
SJSA-1 and U2OS (human OS line) cells were obtained from BeNa Culture Collection (Beijing, China). SISA-1 was grown in Dulbecco’s modified Eagle medium (DMEM) with high glucose (Gibco, Carlsbad, California) and 10% fetal bovine serum (FBS; Gibco). U2OS was cultured in McCoy 5A media (modified with Tricine) containing 10% FBS. All cell incubation was carried out in a humid atmosphere with 5% CO2 at a temperature of 37°C.
Microarray Analysis
RNA extraction was performed by KangChen Bio-tech, Shanghai, China. The human 12 × 135k lncRNA array manufactured by Roche NimbleGen (Roche NimbleGen, Madison, Wisconsin) including protein-coding messenger RNAs (mRNAs) and lncRNAs was used. Approximately 30 586 lncRNAs and 26 109 coding transcripts were collected. Double-strand complementary DNA (ds-cDNA) was synthesized from 5 μg of total RNA using an SuperScript ds-cDNA synthesis kit (Invitrogen, Carlsbad, California). The ds-cDNA was incubated with 4 μg of RNase A at 37°C for 10 minutes and cleaned using phenol. The purified cDNA was quantified using a NanoDrop ND-1000 (Thermo Scientific, Wilmington) and labeled with Cy3. Microarrays were hybridized at 42°C for 16 to 20 hours with 4 µg of Cy3-labeled ds-cDNA in Nimblegen hybridization buffer/hybridization component A in a hybridization chamber (Hybridization System, NimbleGen Systems, Inc). Following hybridization, washing was performed using the Nimblegen wash buffer kit (NimbleGen Systems, Inc). After being washed in an ozone-free environment, the slides were scanned using an Axon GenePix 4000B microarray scanner. The microarray analysis was performed by KangChen Bio-tech, Shanghai, China.
Cell Transfection
The pcDNA3.1 and porcine cytomegalovirus (pCMV) plasmids, SRA1-overexpressing plasmids (pcDNA3.1-SRA1), and SRA1-knockdown plasmids (pCMV-sh-SRA1), as well as inhibitors or mimics specific to miR-208a, were commercially synthesized by GenePharma (Shanghai, China). SJSA-1 and U2OS cells were seeded at 100 000 cells/well in a 6-well plate. After 24 hours, cells were transfected with plasmids (2 μg each hole) or miR-208a inhibitors, mimics, or nontargeting negative control at a final concentration of 50 nM using Lipofectamine 2000 reagent (Invitrogen) following protocols provided by the manufacturer. Cells were collected 48 hours later for further in vitro experiments. Cells without any treatment were recognized as a control group, and cells transfected with pcDNA3.1 vector and pCMV vector were diagnosed as a negative control group.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction Analysis
Total RNA was isolated from tissues or cells using TRIzol reagent (Invitrogen), and cDNA was synthesized with PrimeScript reverse transcriptase (TaKaRa, Kyoto, Japan) and oligo(dT) following the manufacturer’s instructions. The relative expression level of SRA1 was normalized to GAPDH using the 2−ΔΔCt cycle threshold method. Primers were commercially synthesized by GenePharma. The relative amount of SRA1 was normalized to GAPDH, and the relative amounts of miRNAs were normalized to U6. The primers are shown in Table 1.
Table 1.
Quantitative RT-PCR Primers.
| Gene | Primer (5′-3′) |
|---|---|
| MiR-208a | ATAAGACGAGCAAAAAGC |
| MiR-148a | F-TCAGTGCACTACAGAACTTTGT |
| R-GCTGTCAACGATACGCTACGT | |
| MiR-203 | F-GTGCAGGGTCCGAGGT |
| R-GCCGCGTGAAATGTTTAGG | |
| MiR-204 | F-CTGTCACTCGAGCTGCTGGAATG |
| R-ACCGTGTCGTGGAGTCGGCAATT | |
| MiR-211 | F-TCGGCAGGTCCCTTTGTCATCC |
| R-TGCAGGTCAACTGGTGTCGT | |
| MiR-155 | F-CGGGCTTAATGCTAATTGTGA |
| R-CAGCCACAAAAGAGCACAAT | |
| MiR-455-5p | F-CGAGCTTCCTTCTGCAGGT |
| R-CACCACTGCCATCCCACA | |
| MiR-9 | F-TCTTTGGTTATCTAGCTGTATGA |
| R-TGGTGTCGTGGAGTCG | |
| U6 | F-CTCGCTTCGGCAGCACA |
| R-AACGCTTCACGAATTTGCGT | |
| SRA1 | F-GCTGGGCACTGGGAATGTAA |
| R-CACGACCCTACAACCCTCTG | |
| GAPDH | F-AGAAGGCTGGGGCTCATTTG |
| R-GCAGGAGGCATTGCTGATGAT |
Abbreviation: RT-PCR, real-time polymerase chain reaction.
Cell Proliferation Assay With CCK-8
Cell Counting Kit 8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) was utilized to test cell proliferation according to the manufacturer’s protocols. Briefly, 2 × 104/well SJSA-1 and U2SO cells after transfection for 0, 24, 48, and 72 hours were plated in 96-well plate, and 10 µl of CCK-8 was added. After incubation at 37°C for 4 hours, the optimal density (OD) value at 450 nm was calculated with a Bio-Rad 3550 microplate reader (Bio-Rad Laboratories, Hercules, California).
Colony Formation Assay
Transfected cells were diluted and seeded onto 6-well plates with a density of approximately 1000 cells/well. Cells were then incubated at 37°C for 14 days and stained with 0.1% crystal violet (Beyotime, Shanghai) for 30 minutes when colonies were visible. Forty-eight hours later, the plates were photographed, and the colony numbers were counted by a gel documentation system (Bio-Rad).
Cell Invasion and Transwell Assays
Invasion status of cells with differentiated treatments was assessed through transwell assay with Matrigel (BD Biosciences, Bedford, Massachusetts). Cells (1 × 105) were placed in the upper chamber with serum-free DMEM. Then, 600 µl of DMEM with 20% FBS was added to the lower chamber. Forty-eight hours (for invasion) later, the upper side of the membrane was wiped with cotton wool to remove noninvasive cells. Subsequently, the lower surface of the membranes was fixed with 4% paraformaldehyde (Beyotime, Shanghai) and stained using 0.1% crystal violet (Beyotime, Shanghai) at room temperature. Then, 5 fields were picked out randomly, and the number of cells contained in them was counted via a microscope.
Wound-Healing Assay
The migratory ability was assessed through wound-healing assay. Cells (2 × 105) were seeded into a 6-well plate allowed to reach confluence. Then, uniform wounds were scraped using a 200-µl pipette tip across the cell monolayer. Cells were rinsed with phosphate-buffered saline and cultured in the medium. Then, the wound closures were observed after 24 hours. The initial gap length (0 hour) and the residual gap length (24 hours) after wounding were calculated from photomicrographs using an Olympus fluorescence microscope (Olympus).
Cell Apoptosis and Flow Cytometry Analysis
Cells were stained with Annexin-V-FITC according to the manufacturer’s specifications (Molecular Probes, Oregon). Briefly, 3 × 105 cells were cultured in 6-well plates, harvested, and washed. Then, they were resuspended in 100 μl of Annexin-V-binding buffer and incubated with 5 μl of Annexin-V-FITC for 15 minutes at room temperature and counterstained with propidium iodide (final concentration 1 μg/mL). After incubation at room temperature for 15 minutes in the dark, the stained cells were measured using a BD FACSCalibur (BD Bioscience, San Jose, California) as instructed by the manufacturer. The analysis was performed through flow cytometry (BD FACSAria Fusion).
Statistical Analysis
All experiments carried out in this study were done at least 3 times independently. The data were analyzed with Student t test by GraphPad Prism 6.0 (San Diego, California). P <.05 was regarded as statistically significant.
Results
Long Noncoding RNA SRA1 is Downregulated in OS Tissues and Cells
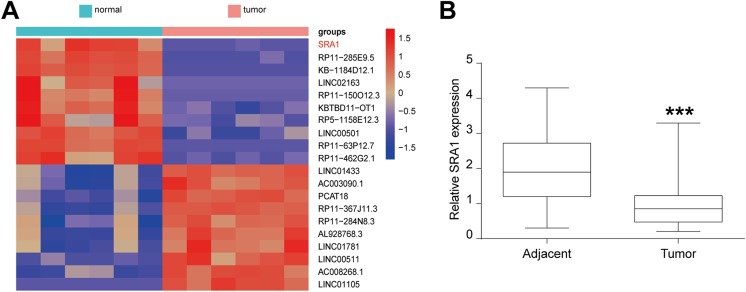
In the hope of determining lncRNAs that were involved in OS tumorigenesis, microarray analysis was utilized. As Figure 1A shows, the heat map revealed that lncRNA SRA1 expression was downregulated in OS tumor tissues compared to that in corresponding adjacent tissues. Then, SRA1 levels in 30 pairs of OS tumor and adjacent tissues were further evaluated and quantified using quantitative real-time polymerase chain reaction (qRT-PCR). As the data of Figure 1B present, lncRNA SRA1 expression was obviously decreased in tumor tissues when compared to that in matched adjacent noncancerous tissues. These findings clearly demonstrate that lncRNA SRA1 was downregulated in OS tissues. In short, the diagram and data uncovered downregulated SRA1 expression in OS tissues, suggesting that SRA1 might act as a tumor inhibitor in OS.
Figure 1.
LncRNA SRA1 expression in osteosarcoma tissues and paired adjacent normal tissues. A, Heat map showing differently expressed lncRNAs in osteosarcoma tissues and paired adjacent normal tissues. Relative expression levels decreased as colors changed from red to purple. B, Relative expression of lncRNA SRA1 in 30 pairs of osteosarcoma tissues (tumor) and adjacent noncancerous tissues (adjacent). Statistics are presented as the mean (SD) of at least 3 independent experiments (** P < .01, *** P < .001). lncRNA indicates long noncoding RNA; SRA1, steroid receptor RNA activator 1; SD, standard deviation.
Restoration of lncRNA SRA1 Inhibits OS Cell Proliferation
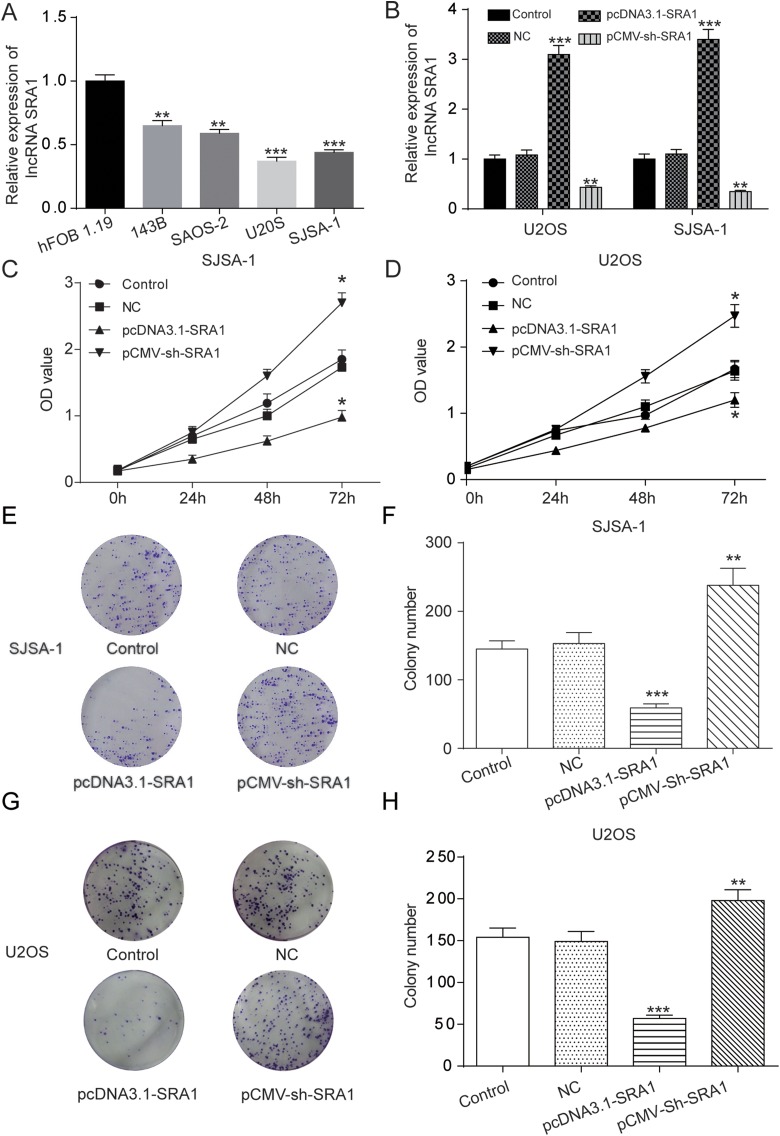
As Figure 2A shows, SRA1 was restrained in 4 OS cell lines, 143B, SAOS-2, U2OS, and SJSA-1, in which the inhibition in U2OS and SISA-1 cells was most apparent. Therefore, U2OS and SJSA-1 were chosen for further study. For the purpose of determining the biological functions of SRA1 in OS development, first, the pcDNA3.1-SRA1, pCMV-sh-SRA1, or empty vectors was transfected in SJSA-1 or U2OS cells. The SRA1 levels after transfection were examined through qRT-PCR system and the results are shown in Figure 2B. Subsequently, in order to determine the influence of SRA1 on cell proliferation in U2OS and SJSA-1, a CCK-8 assay was carried out. The line chart in Figure 2C and D demonstrates that the OD value of pcDNA3.1-SRA1-treated cells was dramatically inhibited while that of pCMV-sh-SRA1-treated cells was significantly increased in comparison with the controlled groups. This indicated that amplification of SRA1 level could suppress OS cell proliferation. Consistently, in colony formation assay, cell proliferation capability of OS cells was also inhibited by the amplification of SRA1. As Figure 2E and H exhibits, pcDNA3.1-SRA1 group formed far fewer colonies than the control group. In contrast, pCMV-sh-SRA1 group formed a large number of colonies. Together, these findings confirmed that lncRNA SRA1 repressed the proliferation of OS cells in vitro.
Figure 2.
Upregulation of lncRNA SRA1 inhibited cell proliferation in osteosarcoma cells. A, qRT-PCR was used to assess the expression of lncRNA SRA1 in different OS cell lines, 143B, SAOS-2, U2OS, and SISA-1 and normal cell lines, hFOB 1.19. B, U2OS and SISA-1 cells were transfected with pcDNA3.1-SRA1 or pCMV-sh-SRA1 and then the SRA1 expression was detected. C, D, CCK-8 assay results showing cell proliferation statuses in control, NC, pcDNA3.1-SRA1, pCMV-sh-SRA1 group at different time points. E-H, Colony formation assay revealing cell proliferation as affected by the manipulation of SRA1 levels in SISA-1 (E, F) and U2OS (G, H) cells. Statistics are presented as the mean (SD) of at least 3 independent experiments (** P < .01, *** P < .001). lncRNA indicates long noncoding RNA; SRA1, steroid receptor RNA activator 1; qRT-PCR, quantitative real-time polymerase chain reaction; OS, osteosarcoma; CCK-8, Cell Counting Kit 8; SD, standard deviation.
Upregulation of lncRNA SRA1 Restrains Cell Migration and Invasion in OS Cells
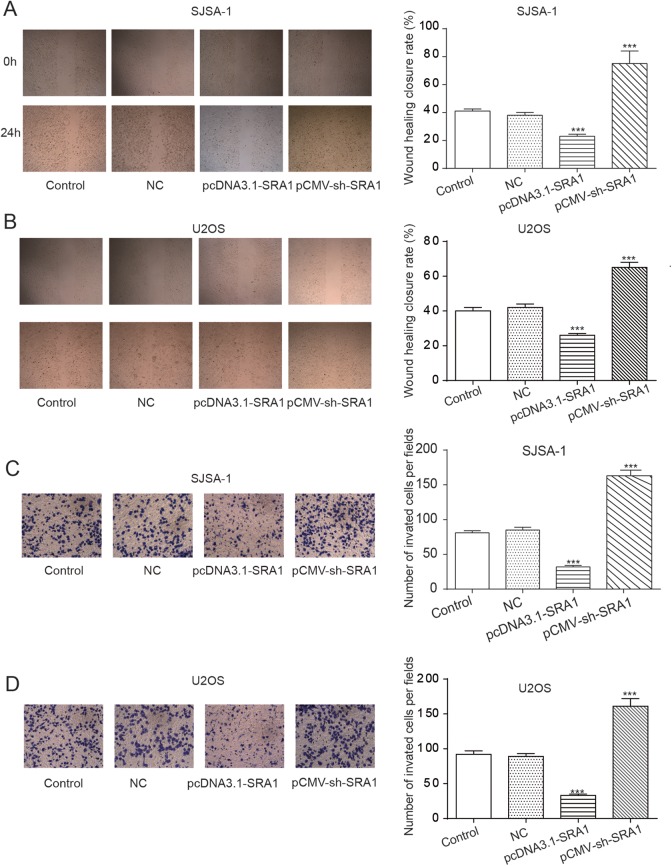
Moving 1 step further, we assessed the role of SRA1 in OS cell migration using wound-healing assay and reviewed cell invasion status by conducting a transwell assay in U2OS and SJSA-1 cell lines. In Figure 3A and B, we found that the percentage of original wound width in the pcDNA3.1-SRA1 group was significantly decreased in comparison with the control, but the closure rate of pCMV-sh-SRA1-treated cells was significantly increased. What was suggested by this result was the inhibition of cell migration by SRA1 overexpression. In terms of cell invasion capability, the transwell assay outcomes shown in Figure 3C and D demonstrate that amplification of lncRNA SRA1 by the transfection of pcDNA3.1-SRA1 dramatically suppressed cell invasion while knockdown of it by the treatment of pCMV-sh-SRA1 significantly facilitated cell invasion. Altogether, these figures suggested the inhibitive function of SRA1 on the migration and invasion of OS cells, U2OS and SJSA-1.
Figure 3.
Upregulation of lncRNA SRA1 inhibited cell migration and invasion of osteosarcoma cells in vitro. A, B, Wound-healing assay result showing cell migration status under the influence of SRA1 in SISA-1 (A) and U2OS (B) cells. C, D, Transwell assay with matrigel demonstrating invasion capability of cells with different SAR1 levels in SISA-1 (C) and U2OS (D) cells. Statistics are presented as the mean (SD) of at least 3 independent experiments (** P < .01, *** P < .001). lncRNA indicates long noncoding RNA; SRA1, steroid receptor RNA activator 1; SD, standard deviation.
Overexpression of lncRNA SRA1 Boosts the Apoptosis Rate in OS Cells
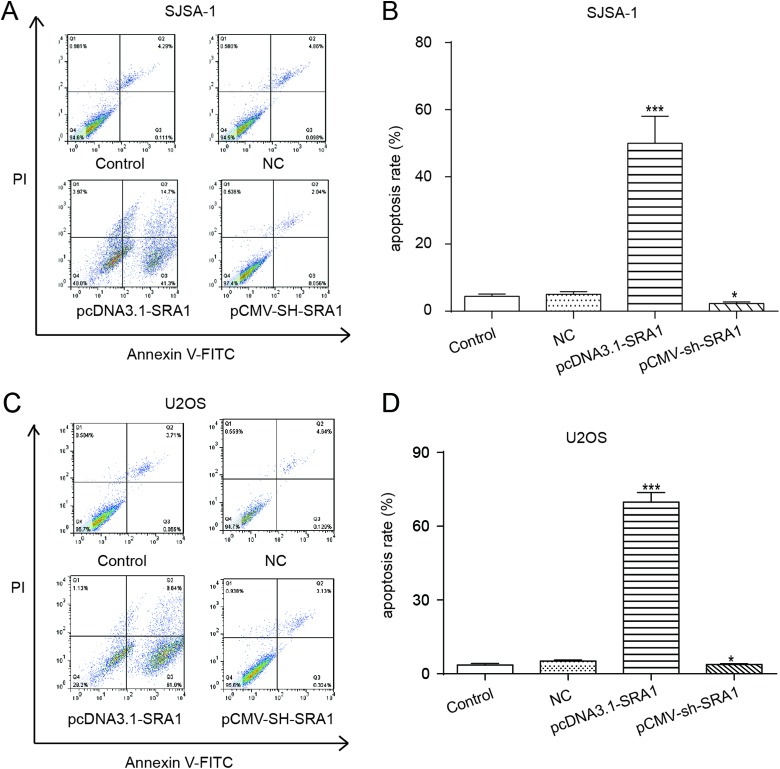
From the flow cytometry analysis results presented in Figure 4A to D, U2OS and SJSA-1 cells with SRA1 reinforcement by the transfection of pcDNA3.1-SRA1 could be observed to exhibit a radical increase in apoptosis rate compared with controlled cells. In contrast, pCMV-sh-SRA1 cells showed markedly inhibited apoptotic cells. These results revealed that overexpression of lncRNA SRA1 promoted apoptosis in OS cells.
Figure 4.
Overexpression of lncRNA SRA1 facilitated apoptosis in osteosarcoma cells. A-D, were flow cytometry analysis of the effects of lncRNA SRA1 overexpression on SJSA-1 (A, B) and U2OS (C, D) cells apoptosis. Statistics are presented as the mean (SD) of at least 3 independent experiments (** P < .01, *** P < .001). lncRNA indicates long noncoding RNA; SRA1, steroid receptor RNA activator 1; SD, standard deviation.
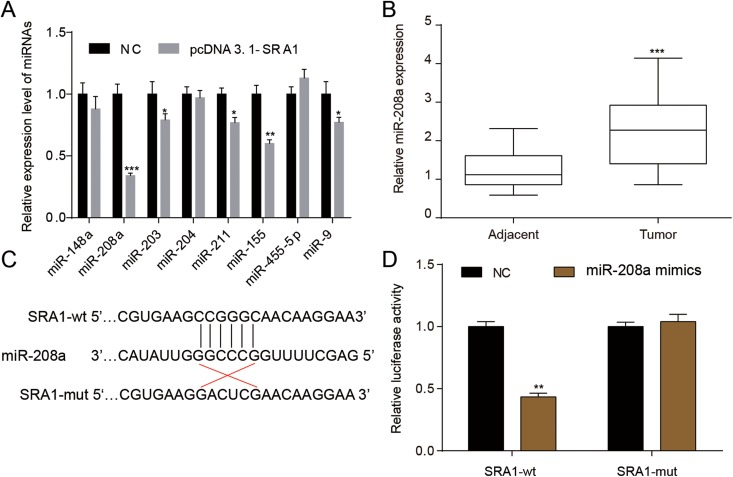
MiR-208a Is Suppressed When Enhancing SRA1
To investigate the molecular regulation mechanism of lncRNA SRA1, pcDNA3.1-SRA1 was employed in silencing SRA1 followed by detecting the dysregulating miRNAs that were predicted to target lncRNA SRA1 via miRcode (www.mircode.org). As shown in Figure 5A, 5 miRNAs were significantly attenuated, and the decrease in miR-208a was more evident than others. Furthermore, qRT-PCR assays demonstrated that miR-208a was upregulated in tumor tissues compared with adjacent tissues, as expected (Figure 5B). In Figure 5C and D, dual-luciferase experiment was utilized to validate the targeting relationship between lncRNA SRA1 and miR-208a further. Therefore, we hypothesized that lncRNA SRA1 are involved in the progression of OS via modulating miR-208a.
Figure 5.
LncRNA SRA1 targeted miR-208a. A, Cells were transfected with pcDNA3.1-SRA1, and then the predicted miRNAs targeted by lncRNA SRA1 was detected. B, MiR-208a relative expression was assessed by qRT-PCR in adjacent or tumor tissues. C, The SRA1 wild-type or mutant type and miR-208a sequence were shown. D, Dual-luciferase assay was exerted to investigate the relationship between SRA1 and miR-208a. Statistics are presented as the mean (SD) of at least 3 independent experiments (* P < .05, ** P < .01, *** P < .001). lncRNA indicates long noncoding RNA; SRA1, steroid receptor RNA activator 1; miRNA, microRNA; qRT-PCR, quantitative real-time polymerase chain reaction; SD, standard deviation.
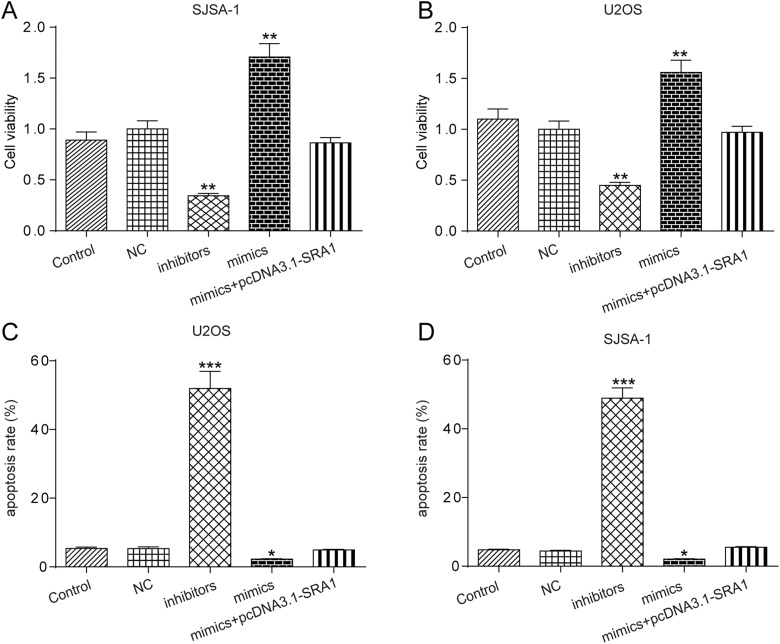
Long Noncoding RNA SRA1-Targeting miR-208a Modulates the Cell Physiological State
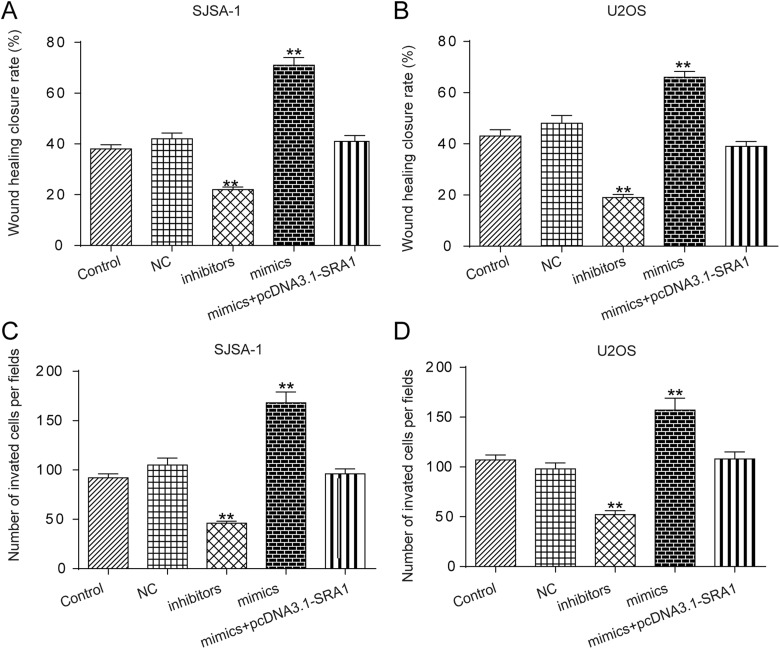
In the following experiments, inhibitors, mimics specific to miR-208a, and mimics+pcDNA3.1-SRA1 were independently transfected into SJSA-1 or U2OS cell lines. Figure 6A and B demonstrates that cell viability was restrained by inhibitors, but significantly enhanced by mimics. In addition, the effects of mimics were reversed by cotransfected with mimics and pcDNA3.1-SRA1. Similarly, cell apoptosis in SISA-1 and U2OS cell was promoted by inhibitors but hindered by mimics, which was reversed by upregulating SRA1 expression (Figure 6C and D). In addition, Figure 7A and B suggests that wound-healing closure rate of SISA-1 and U2OS cells was suppressed by inhibitors, while mimics showed opposite roles. If mimics and pcDNA3.1-SRA1 were cotransfected into cells, the acceleration of cell migration resulting from mimics would be decreased significantly. Furthermore, from the transwell invasion assays results presented in Figure 7C and D, mimics significantly increased the number of invasive cells of SISA-1 and U2OS cells. When cotransfected pcDNA3.1-SRA1 and mimics, the positive effect of mimics was reversed. All the results showed that lncRNA SRA1 regulated the progress of OS via sponging miR208a.
Figure 6.
LncRNA SRA1 targeting miR-208a was modulated cell viability and apoptosis. A, B, CCK-8 assay results showing cell proliferation statuses in control, NC, inhibitors, mimics, and mimics+pcDNA3.1-SRA1 group. C, D, Flow cytometry analysis of the effects of dysregulation of miR-208a in SJSA-1 and U2OS cells on apoptosis. Statistics are presented as the mean (SD) of at least 3 independent experiments (* P < .05, ** P < .01, *** P < .001). lncRNA indicates long noncoding RNA; SRA1, steroid receptor RNA activator 1; miRNA, microRNA; CCK-8, Cell Counting Kit 8; SD, standard deviation.
Figure 7.
LncRNA SRA1 targeting miR-208a was modulated cell migration and invasion. A, B, Wound-healing assay result showing cell migration status in SISA-1 (A) and U2OS (B) cells. C, D, Transwell assay with matrigel demonstrating invasion capability of cells in SISA-1 (C) and U2OS (D) cells. Statistics are presented as the mean (SD) of at least 3 independent experiments (** P < .01, *** P < .001). lncRNA indicates long noncoding RNA; SRA1, steroid receptor RNA activator 1; miRNA, microRNA; SD, standard deviation.
Discussion
Osteosarcoma originates in the mesenchymal tissue and is a prevalent primary solid malignancy of the bone, affecting children and adolescents, especially during a growth spurt. Cancer metastasis and recurrence are 2 primary reasons accounting for the high death rate of OS.19 It has frequently been proved that lncRNAs are intimately involved in carcinogenesis and tumorigenesis of various malignancies including OS.20 This study thus investigated the molecular factor that might affect OS proliferation and apoptosis.
For this purpose, we first carried out a microarray assay to confirm lncRNAs that were abnormally expressed in OS. In previous studies, a number of lncRNAs were already identified to be aberrantly expressed in OS cells. For instance, lncRNA prostate cancer–associated transcript 1,21 urothelial cancer associated 1,22 Angelman syndrome chromosome region,23 and MALAT124 were upregulated in OS tissues when compared to normal adjacent tissues, indicating their oncogenic role in OS. Other lncRNAs, however, were validated to be downregulated in OS, demonstrating inhibitive capability in tumorigenesis and tumor progression, such as LncRNA growth arrest specific 5,25 MALAT1,26 and X inactive specific transcript.27 In this study, the microarray experiment demonstrated that lncRNA SRA1 was the most significantly knocked down lncRNA among all profiled lncRNAs. This result was later supported by qRT-PCR analysis, showing downregulation of lncRNA SRA1 in OS tumor tissues compared to paired normal tissues. Steroid receptor RNA activator (SRA) was initially identified in a human B-lymphocyte library.28 Later, the products of the SRA gene were indicted to affect tumorigenesis.29 The previous study uncovered decreased expression of SRA1 in hepatocellular carcinoma tissues compared to corresponding noncancerous tissues.13 In the study of Lin et al, low expression levels of SRA lncRNA were also detected in ovarian endometriotic tissues.30 These findings were in agreement with ours. Therefore, we assumed that SRA1 might play a tumor-suppressive role in OS, and we conducted experiments on cell migration, invasion, proliferation, and apoptosis to testify this assumption.
It was revealed in one study that SRA promoted apoptotic activities and cell proliferation in vivo.29 Consistently, flow cytometry analysis in this study also showed that apoptosis rate of cell lines with SRA1 overexpressed was apparently increased. Another study highlighted that the depletion of SRA/SRAP as a whole in MDA-MB-231 breast cancer cells decreased cell migration ability as a whole, but it did not provide any information as to whether cell migration was affected by SRA1 depletion alone.31 The present study investigated for the first time the impact of SRA1 knockout on cell migration in OS. Transwell results demonstrated that SRA1 suppression dramatically contributed to cell migration as well as cell invasion in OS. Additionally, we found that lncRNA SRA1 functioned as a restraining factor in the proliferation of OS cells.
To obtain a comprehensive understanding of the suppressive role of lncRNA SRA1 in OS, a series of experiments was conducted to find its targeting gene and reveal the molecular mechanism. In our study, lncRNA SRA1 sponged miR-208a to suppress its expression involved in regulating the cell migration, viability, and apoptosis in OS. In a previous study, lncRNA miR210HG sponged miR-503, resulting in facilitating OS cell invasion and migration in OS.32 Furthermore, Xie et al demonstrated that lncRNA TUG1 could target miR-9 and then modulate POU2F1 expression, which contributed to tumorigenesis of OS.33 These researchers suggested that the endogenous regulatory mechanism of lncRNA-miRNA played an essential role in OS development.
LncRNA SRA1 played an antitumor role in OS via sponging miR-208a as it reduced cell migration, invasion, and proliferation while facilitating cell apoptosis. This provided a new possible target for the treatment of OS.
Abbreviations
- CCK-8
Cell Counting Kit 8
- CMV
cytomegalovirus
- ds-cDNA
double-strand complementary DNA
- DMEM
Dulbecco’s modified Eagle medium
- FBS
fetal bovine serum
- LncRNA
long noncoding RNA
- MALAT1
metastasis-associated lung adenocarcinoma transcript 1
- mRNAs
messenger RNAs
- OS
osteosarcoma
- qRT-PCR
quantitative real-time polymerase chain reaction
- SRA
steroid receptor RNA activator
- SRA1
steroid receptor RNA activator 1.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (No. 81302336).
ORCID iD: Ming Zhang, MD  https://orcid.org/0000-0002-2774-7839
https://orcid.org/0000-0002-2774-7839
References
- 1. Cepeda MLA, Sosa AJ, Mora G. Telangiectatic osteosarcoma in an infant [Mexican]. Bol Med Hosp Infant Mex. 2017;74(1):60–64. [DOI] [PubMed] [Google Scholar]
- 2. Taran SJ, Taran R, Malipatil NB. Pediatric osteosarcoma: an updated review. Indian J Med Paediatr Oncol. 2017;38(1):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang L, Shrestha S, LaChaud G, Scott MA, James AW. Review of microRNA in osteosarcoma and chondrosarcoma. Med Oncol. 2015;32(6):613. [DOI] [PubMed] [Google Scholar]
- 4. Evola FR, Costarella L, Pavone V, et al. Biomarkers of osteosarcoma, chondrosarcoma, and Ewing sarcoma. Front Pharmacol. 2017;8:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friebele JC, Peck J, Pan X, Abdel-Rasoul M, Mayerson JL. Osteosarcoma: a meta-analysis and review of the literature. Am J Orthop (Belle Mead NJ). 2015;44(12):547–553. [PubMed] [Google Scholar]
- 6. Greco S, Zaccagnini G, Perfetti A, et al. Long noncoding RNA dysregulation in ischemic heart failure. J Transl Med. 2016;14(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sayad A, Hajifathali A, Hamidieh AA, Esfandi F, Taheri M. Fas-antisense long noncoding RNA and acute myeloid leukemia: is there any relation? Asian Pac J Cancer Prev. 2018;19(1):45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geisler S, Coller J. RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol. 2013;14(11):699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li F, Cao L, Hang D, Wang F, Wang Q. Long non-coding RNA HOTTIP is up-regulated and associated with poor prognosis in patients with osteosarcoma. Int J Clin Exp Pathol. 2015;8(9):11414–11420. [PMC free article] [PubMed] [Google Scholar]
- 10. Sun XH, Yang LB, Geng XL, Wang R, Zhang ZC. Increased expression of lncRNA HULC indicates a poor prognosis and promotes cell metastasis in osteosarcoma. Int J Clin Exp Pathol. 2015;8(3):2994–3000. [PMC free article] [PubMed] [Google Scholar]
- 11. Luo W, He H, Xiao W, et al. MALAT1 promotes osteosarcoma development by targeting TGFA via MIR376A. Oncotarget. 2016;7(34):54733–54743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu S, Sheng L, Miao H, et al. SRA gene knockout protects against diet-induced obesity and improves glucose tolerance. J Biol Chem. 2014;289(19):13000–13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luo P, Jing W, Zhu M, et al. Decreased expression of lncRNA SRA1 in hepatocellular carcinoma and its clinical significance. Cancer Biomark. 2017;18(3):285–290. [DOI] [PubMed] [Google Scholar]
- 14. Hube F, Velasco G, Rollin J, Furling D, Francastel C. Steroid receptor RNA activator protein binds to and counteracts SRA RNA-mediated activation of MyoD and muscle differentiation. Nucleic Acids Res. 2011;39(2):513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao Y, Ma X, Yao Y, et al. miR-155 regulates the proliferation and invasion of clear cell renal cell carcinoma cells by targeting E2F2. Oncotarget. 2016;7(15):20324–20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu SL, Lee DC, Sohn HA, et al. Homeobox A9 directly targeted by miR-196b regulates aggressiveness through nuclear factor-kappa B activity in non-small cell lung cancer cells. Mol Carcinog. 2016;55(12):1915–1926. [DOI] [PubMed] [Google Scholar]
- 17. Cui HB, Ge HE, Wang YS, Bai XY. miR-208a enhances cell proliferation and invasion of gastric cancer by targeting SFRP1 and negatively regulating MEG3. Int J Biochem Cell Biol. 2018;102:31–39. [DOI] [PubMed] [Google Scholar]
- 18. Yin K, Liu M, Zhang M, et al. miR-208a-3p suppresses cell apoptosis by targeting PDCD4 in gastric cancer. Oncotarget. 2016;7(41):67321–67332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He Y, Ma J, Wang A, et al. A support vector machine and a random forest classifier indicates a 15-miRNA set related to osteosarcoma recurrence. OncoTargets Ther. 2018;11:253–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9(6):703–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang X, Zhang Y, Mao Y, Ma X. The lncRNA PCAT1 is correlated with poor prognosis and promotes cell proliferation, invasion, migration and EMT in osteosarcoma. OncoTargets Ther. 2018;11:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li T, Xiao Y, Huang T. HIF1alphainduced upregulation of lncRNA UCA1 promotes cell growth in osteosarcoma by inactivating the PTEN/Akt signaling pathway. Oncol Rep. 2018;39(3):1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang F, Peng H. LncRNA-ANCR regulates the cell growth of osteosarcoma by interacting with EZH2 and affecting the expression of p21 and p27. J Orthop Surg Res. 2017;12(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Y, Zhang Y, Yang T, et al. Long non-coding RNA MALAT1 for promoting metastasis and proliferation by acting as a ceRNA of miR-144-3p in osteosarcoma cells. Oncotarget. 2017;8(35):59417–59434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Y, Kong D. LncRNA GAS5 represses osteosarcoma cells growth and metastasis via sponging miR-203a. Cell Physiol Biochem. 2018;45(2):844–855. [DOI] [PubMed] [Google Scholar]
- 26. Zhang ZC, Tang C, Dong Y, et al. Targeting the long noncoding RNA MALAT1 blocks the pro-angiogenic effects of osteosarcoma and suppresses tumour growth. Int J Biol Sci. 2017;13(11):1398–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang R, Xia T. Long non-coding RNA XIST regulates PDCD4 expression by interacting with miR-21-5p and inhibits osteosarcoma cell growth and metastasis. Int J Oncol. 2017;51(5):1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lanz RB, McKenna NJ, Onate SA, et al. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell. 1999;97(1):17–27. [DOI] [PubMed] [Google Scholar]
- 29. Lanz RB, Chua SS, Barron N, Soder BM, DeMayo F, O’Malley BW. Steroid receptor RNA activator stimulates proliferation as well as apoptosis in vivo. Mol Cell Biol. 2003;23(20):7163–7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin K, Zhan H, Ma J, et al. Silencing of SRA1 regulates ER expression and attenuates the growth of stromal cells in ovarian endometriosis. Reprod Sci. 2017;24(6):836–843. [DOI] [PubMed] [Google Scholar]
- 31. Yan Y, Cooper C, Hamedani MK, et al. The steroid receptor RNA activator protein (SRAP) controls cancer cell migration/motility. FEBS Lett. 2015;589(24 pt B):4010–4018. [DOI] [PubMed] [Google Scholar]
- 32. Li J, Wu QM, Wang XQ, Zhang CQ. Long noncoding RNA miR210HG sponges miR-503 to facilitate osteosarcoma cell invasion and metastasis. DNA Cell Biol. 2017;36(12):1117–1125. [DOI] [PubMed] [Google Scholar]
- 33. Xie CH, Cao YM, Huang Y, et al. Long non-coding RNA TUG1 contributes to tumorigenesis of human osteosarcoma by sponging miR-9-5p and regulating POU2F1 expression. Tumour Biol. 2016;37(11):15031–15041. [DOI] [PubMed] [Google Scholar]