Figure 3.
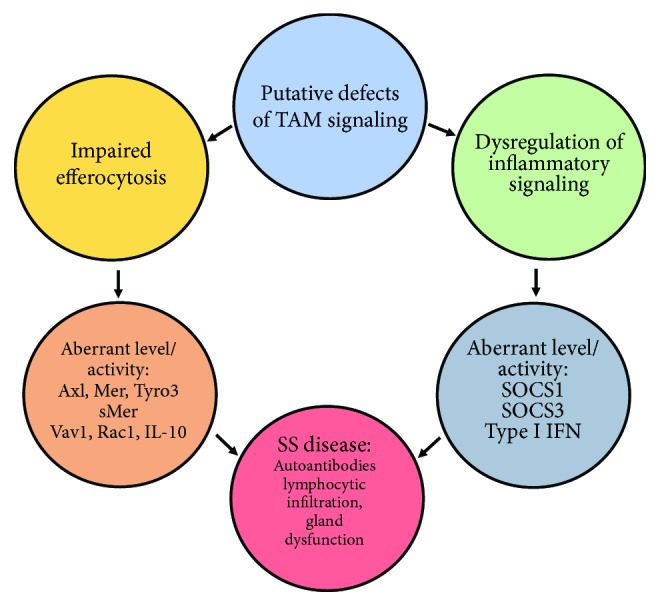
Summary of potential contributions of defective TAM signaling to SS. The etiology of SS is multifactorial, but it is known that innate immune dysfunction precedes adaptive immune dysfunction in the salivary glands. Here, we hypothesize that the TAM family of tyrosine kinases is involved in SS pathology through the TAM-mediated efferocytosis and Type I IFN regulatory pathways. We speculate that aberrations in TAM expression coupled with increased soluble Mer may account for the reported efferocytosis impairment, while downstream elements of efferocytosis signaling including Vav1 and Rac1 activation are unknown, as is SS macrophage response to IL-10 in the context of efferocytosis. Furthermore, we suggest that dysregulation of SOCS 1 and SOCS3 expression and activity may contribute to the overactive IFN signaling observed in SS. We postulate that these two failures in TAM signaling may be initial events in SS pathology that eventually lead to autoantibody generation, lymphocytic infiltration, and gland secretory dysfunction.
