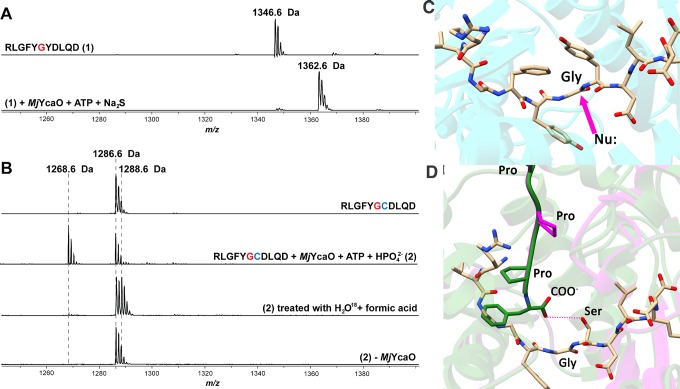
Figure 5.
MALDI-TOF-MS analysis of MjMcrA 11-mer peptide thioamidation and MjMcrA–Y(+1)C 11-mer cyclodehydration. The sequences of the McrA 11-mer variants with the site for thioamidation (Gly(0)) in red, and the Cys(+1) replacement in blue. (A) Thioamidation catalyzed by MjYcaO. Top: mass spectrum of the unmodified MjMcrA 11-mer peptide, m/z 1346.6 Da. Bottom: mass spectrum of the MjMcrA 11-mer peptide after reacting with MjYcaO, Na2S, and ATP, showing the thioamidated product, m/z 1362.6 Da. (B) Cyclodehydration catalyzed by MjYcaO. From top to bottom: mass spectrum of the unmodified MjMcrA–Y(+1)C 11-mer peptide; MjMcrA–Y(+1)C 11-mer treated with MjYcaO and ATP in the presence of phosphate; acid hydrolysis of cyclodehydrated MjMcrA–Y(+1)C 11-mer peptide in [18O]-labeled water; control where MjYcaO is omitted. (C) View of the MjYcaO (in cyan) bound to MjMcrA (shown as tan sticks). The site of nucleophilic attack by the sulfur source is shown with a purple arrow. (D) Superposition of the computational structure of PatD (green) and TruD crystal structure (purple) with the MjMcrA 11-mer peptide structure (shown as tan sticks) modeled in the active site. In the peptide, Tyr(+1) has been modified to a Ser for presentation.