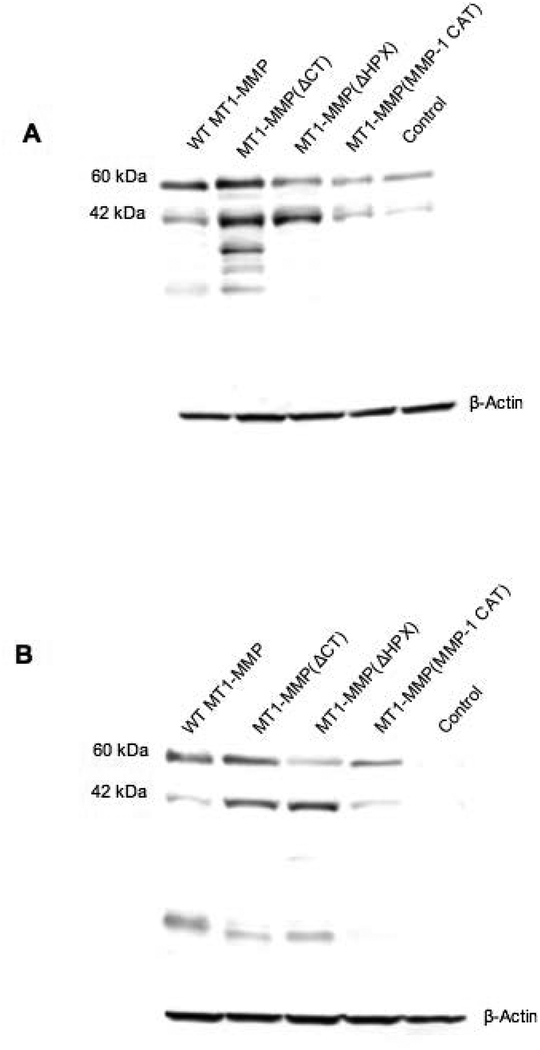
Figure 3.
Western blotting of transfected MCF-7 cells. MCF-7 cells stably transfected with the original pcDNA3.1 plasmid (Control) or WT-MT1-MMP, MT1-MMP(ΔCT), MT1-MMP(ΔHPX), or MT1-MMP(MMP-1 CAT) were lysed and samples identified by Western blotting with anti-MT1-MMP (A) CAT domain mAb or (B) hinge domain mAb. Western blot analysis showed the active (63 kDa) and autodegraded (42 kDa) forms of WT-MT1-MMP or MT1-MMP(ΔCT), as well as the active forms of MT1-MMP(MMP-1 CAT) (56 kDa) or MT1-MMP(ΔHPX) (42 kDa). In the case of MT1-MMP(ΔHPX), the protein observed at 63 kDa is natural production of WT-MT1-MMP by MCF-7 cells. The lowest MW degradation product in the WT-MT1-MMP and MT1-MMP(ΔCT) samples (~18 kDa) probably corresponds to the autocatalytically generated Tyr112-Ala255 MT1-MMP, which is inactive and does not bind TIMP-2 (101). The other degradation products observed in the MT1-MMP(ΔCT) sample (MW ~31–35 kDa) may be related to non-autocatalytic processing within the HPX domain (101). These fragments would be released from the cell surface (49). Results shown are representative of three or more experiments performed. As observed previously, WT-MT1-MMP, MT1-MMP(ΔCT), and MT1-MMP(MMP-1 CAT) exhibit similar mobility in Western blot analysis (28, 41). The loading control was β-actin.