Figure 4.
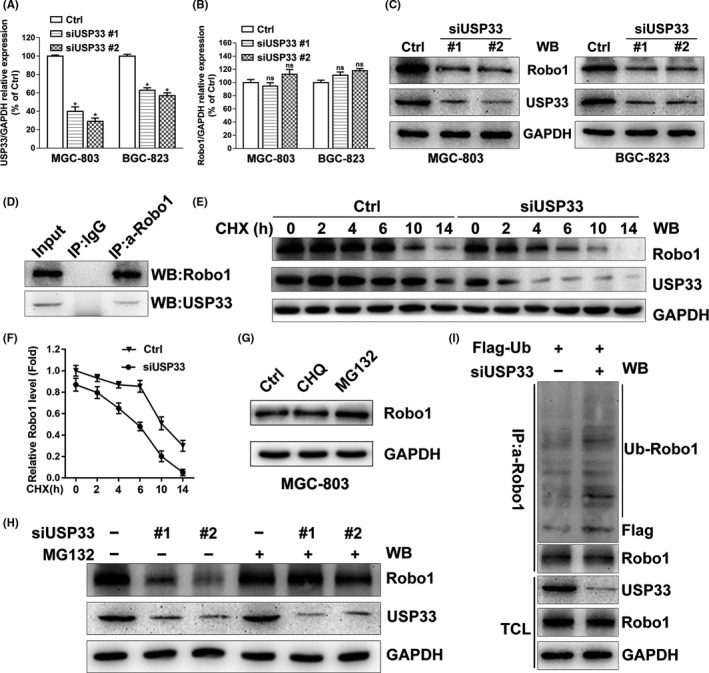
USP33 interacts with Robo1 and increases the stability of Robo1 by deubiquitinating Robo1 in GC cells. A, Relative USP33 mRNA levels in MGC‐803 and BGC‐823 transfected with control siRNA, siUSP33 #1 or siUSP33 #2 were examined by qRT‐PCR. B, Relative Robo1 mRNA levels in MGC‐803 and BGC‐823 cells transfected with control siRNA or siUSP33. C, Western blotting showed the protein levels of USP33 and Robo1 in MGC‐803 and BGC‐823 cells transfected with control siRNA or siUSP33. D, Interaction of the Robo1 and USP33 proteins in MGC‐803 cells. Co‐immunoprecipitation was performed using either control IgG or anti‐Robo1 antibody. Immunoprecipitated proteins were detected by Western blotting using anti‐Robo1 and anti‐USP33. E, MGC‐803 cells were transfected with control siRNA or siUSP33 and treated with cycloheximide (CHX, 50 μg/mL) for different periods of time. The Robo1 and USP33 protein levels were subjected to Western blotting analysis. F, Quantification of relative Robo1 protein levels. G, MGC‐803 cells were left untreated or treated with chloroquine (CHQ, 50 μmol/L, 10 h) or MG132 (10 μmol/L, 10 h), and Robo1 was detected by Western blotting. H, MGC‐803 cells transfected with control siRNA or siUSP33 were treated with MG132 (10 μmol/L) or untreated, 10 hours later, and Robo1 and USP33 were detected from the cell lysate. I, Robo1 ubiquitination was examined in MGC‐803 cells co‐transfected with Flag‐ubiquitin, control siRNA or siUSP33. Co‐immunoprecipitation was carried out with anti‐Robo1 after treated with MG132 (10 μmol/L, 10 h) and then examined by Western blotting. TCL: Total cell lysate. All Western blotting analyses in this figure 4 were using GAPDH as the internal control
