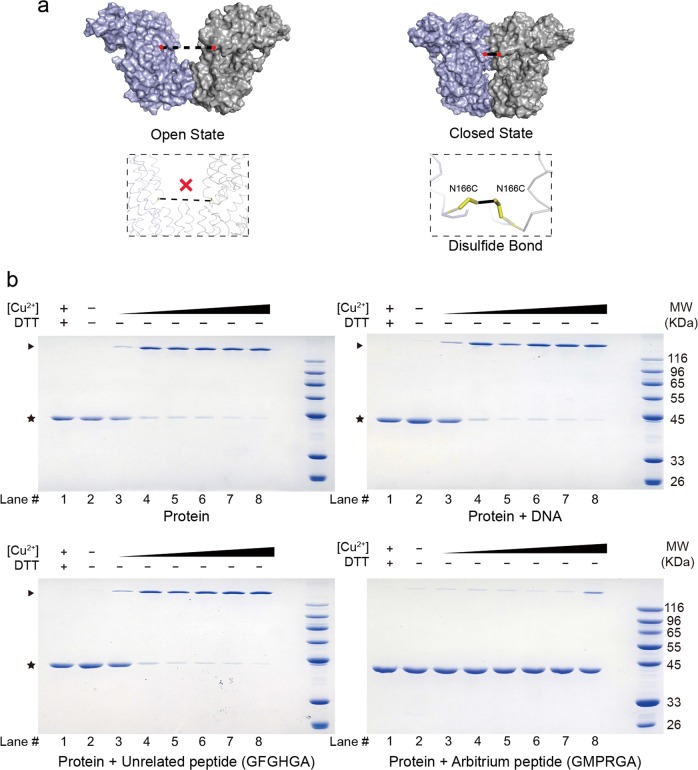
Fig. 6. The arbitrium peptide stabilizes the AimR dimer in the open state.
a Dimeric interface II is mediated by residues N166 in the closed state of AimR. Asn166 of each protomer was mutated to Cys for disulfide bond formation. Both residues are labeled red. b Crosslinking of AimR-N166C. Left upper panel: Crosslinking of AimR-N166C with increasing concentrations of o-phenanthroline copper complex (Cu2+). The reactants were subjected to nonreducing SDS-PAGE followed by Coomassie blue staining. Right upper panel: Crosslinking of AimR-N166C with increasing concentrations of Cu2+ in the presence of DNA. Left lower panel: Crosslinking of AimR-N166C with increasing concentrations of Cu2+ in the presence of an unrelated peptide. Right lower panel: Crosslinking of AimR-N166C with increasing concentrations of Cu2+ in the presence of the arbitrium peptide. The highest concentration of Cu2+ in lane 8 is 1 mM, and the concentration decreased sequentially by a 1:3 gradient from lane 8 to lane 3. The addition of 5 mM DTT effectively broke the disulfide bond (Lane 1)