Abstract
Potassium channels are the most diverse group of ion channels in humans. They take vital parts in numerous physiological processes and their malfunction gives rise to a range of pathologies. In addition to small molecules, there is a wide selection of several hundred polypeptide ligands binding to potassium channels, the majority of which have been isolated from animal venoms. Until recently, only scorpion toxins received focused attention being systematically assembled in the manually curated Kalium database, but there is a diversity of well-characterized potassium channel ligands originating from other sources. To address this issue, here we present the updated and improved Kalium 2.0 that covers virtually all known polypeptide ligands of potassium channels and reviews all available pharmacological data. In addition to an expansion, we have introduced several new features to the database including posttranslational modification annotation, indication of ligand mode of action, BLAST search, and possibility of data export.
Subject terms: Ion channels in the nervous system, Potassium channels, Receptor pharmacology, Protein databases
| Design Type(s) | database creation objective • data integration objective |
| Measurement Type(s) | ligand |
| Technology Type(s) | digital curation |
| Factor Type(s) | Species |
| Sample Characteristic(s) | Serpentes • Scorpiones • Araneae • Anemone • Conus <genus> • Ancylostoma ceylanicum • Anoplius samariensis • Apis mellifera • Brugia malayi • Heloderma horridum horridum • Homo sapiens • Pseudoplectania nigrella • Scolopendra mutilans • Scolopendra subspinipes dehaani |
Machine-accessible metadata file describing the reported data (ISA-Tab format)
Background & Summary
Potassium (K+) channels are a superfamily of integral membrane proteins responsible for selective potassium ion permeation through cell membranes. Activity of K+ channels regulates cell excitability and controls the shape of the action potential1. Being present in various cells they participate in processes as diverse as cognition, muscle contraction, and hormone secretion2. K+ channels are composed of two or four major α subunits that form the pore and auxiliary β subunits3,4. K+ channels of mammals are classified into four groups according to gene homology and structure of the α subunits: calcium- and sodium-activated (KCa and KNa), inwardly rectifying (Kir), two pore domain (K2P), and voltage-gated (KV) potassium channels5–9.
A large number of various molecules can interact with K+ channels. Three major classes are often cited: metal ions, low-molecular-mass substances, and polypeptides10. Despite structural differences most K+ channel ligands may either physically occlude the channel pore, or change channel properties through gating modification11. Polypeptide ligands are of special interest to researchers due to high affinity (often active at nanomolar or even subnanomolar concentrations) and selectivity towards their targets. Most of these molecules are toxins from venomous animals but some are found in different sources12–15. Polypeptide ligands play a key role in unravelling the functions of K+ channels and serve a pool of natural prototypes for drug discovery16.
>95% of K+ channel polypeptide ligands have been identified in just five groups of organisms10 and scorpion toxins (KTx) provide >50% of this variability. They consist of ∼20–75 amino acid residues and usually contain 2–4 disulfide bridges17. Five structural folds are described for KTx: cysteine-stabilized α-helix/β-sheet (CSα/β), cysteine-stabilized helix-loop-helix (CSα/α) with two or three disulfide bonds, Kunitz-type, and inhibitor cystine knot (ICK) folds18. KTx generally inhibit KV and KCa channels through pore blockage10. The most famous ligands of K+ channels from snakes are dendrotoxins that contain ∼55–60 amino acid residues and form a Kunitz-type fold19,20. Another important group is myotoxin-like polypeptides composed of ∼40–50 amino acids, which assume a similar fold to human β-defensins and display versatile activities including KV channel inhibition21. Spider toxins containing ∼30–40 amino acid residues and forming the ICK motif inhibit mostly K+ channel activation via interactions with the voltage sensor22. The founding member of this group is hanatoxin23 and their peculiar ability is to partition into membranes and interact with the channels by lateral association within the membranes22,24. Some weak pore blockers of KV channels assuming the Kunitz-type fold have also been found in spider venom25. K+ channel ligands from sea anemones are composed of ∼35–65 amino acid residues and can be subdivided into three subgroups by structural features26. Their spatial structures are presented by a combination of α and/or 310-helices, several β-strands, or the Kunitz-type fold18,26. Sea anemone toxins often bear posttranslational modifications and inhibit KV and KCa channels10. Cone snails use a number of different structural classes of toxin to target KV channels: κA-, κO-, κM-, κI-, κJ-, and κL-conotoxins27,28. These polypeptides comprise ∼20–30 amino acid residues and present diverse disulfide patterns and folds29. Two toxins have a particularly unusual structure: conkunitzin-S1, a 60 residues-long polypeptide with the Kunitz-type fold30, and contryphan-Vn of just nine amino acids31. Conotoxins are also often subjected to posttranslational modifications. In addition, a comparatively small number of molecules affecting K+ channels has been found in some species of bees, worms, lizards, fungi, and scolopendra13,14,32–34; moreover, human β-defensin 4A displays activity against several KV isoforms15.
The first version of Kalium comprised only scorpion toxins35, while its current expansion and update includes all known polypeptide ligands identified in living organisms. For all these compounds detailed activity data are provided collected from original manuscripts. Several major improvements have been introduced, such as the indication of toxin mode of action, BLAST search, and possibility to export data in .csv (comma-separated) or .txt (tab-delimited) format. Kalium is manually curated, and presents a comprehensive list of all known polypeptide K+ channel ligands available to users. Kalium is of primary utility to researchers investigating the structure and function of K+ channels, toxinologists addressing the variability and mode of action of natural toxins, pharmacologists and research and development managers involved in drug discovery targeting K+ channels, and biochemical community in general.
Methods
Data sources and curation
Data for Kalium 2.0 were assembled from scorpion venom peptide entries already present in the first release of Kalium35, which was updated and expanded by adding the available information on K+ channel ligands from other organisms. As a result, Kalium 2.0 contains twice as many entries as Kalium 1.0. The compiled data on all publically available sequences of polypeptide ligands of K+ channels were obtained from UniProt (http://www.uniprot.org/)36. Available PDB structures with links to the RCSB Protein Data Bank (https://www.rcsb.org)37 and location of disulfide bonds were also extracted from UniProt. The data set was then manually filtered and refined, including the following steps: removal of peptides with partial sequence, removal of entries supported by genomic or transcriptomic information only, and sorting by the source organism into six groups: snakes, scorpions, spiders, sea anemones, cone snails, and miscellaneous. Kalium 1.0 and 2.0 entries statistics is summarized in Table 1.
Table 1.
Kalium entries statistics.
| Source organisms | Entries added | Current number of entries | ||
|---|---|---|---|---|
| Kalium 1.0 | Kalium 2.0 | |||
| Scorpions | 174 | 19 | 193 | |
| Snakes | — | 29 | 29 | |
| Spiders | — | 50 | 50 | |
| Sea anemones | — | 35 | 35 | |
| Cone snails | — | 19 | 19 | |
| Miscellaneous | Nematodes | — | 2 | 2 |
| Hymenopterans | — | 4 | 4 | |
| Lizards | — | 1 | 1 | |
| Human | — | 1 | 1 | |
| Fungi | — | 1 | 1 | |
| Centipedes | — | 5 | 5 | |
| Total | 174 | 164 | 340 | |
Partially sequenced polypeptides were excluded because they cannot be used straightforwardly for nomenclature or in further research and bring confusion to the entire data set. Sequences obtained from transcriptomes without verification on protein level were also left out because (i) they are of less interest for researchers, (ii) there is differential presence or absence of transcriptomic entries from different organisms in UniProt-supported toxin classification and (iii) transcriptomic studies grow fast in numbers and often provide data of low accuracy.
In many cases, experimentally measured molecular masses for natural polypeptides are unavailable. For this reason, molecular masses were calculated for every curated Kalium 2.0 entry. Commonly, the task of precise molecular mass calculation is more complicated than it seems to be, due to co- and posttranslational modifications. In addition to the more widespread cleavage of signal and propeptides, N-terminal cyclization of glutamine, C-terminal amidation, and disulfide bridge formation, as an improvement in Kalium 2.0 we also took into consideration the following modifications: Nε-formylation of lysine, γ-carboxylation of glutamic acid, and γ-hydroxylation of proline. Tables of amino acid masses and modifications from the FindMod tool of the ExPASy server38,39 were used for calculations:
https://web.expasy.org/findmod/findmod_masses.html#aas — amino acid molecular masses,
https://web.expasy.org/findmod/PYRRE.html — cyclization of N-terminal glutamine into pyroglutamate,
https://web.expasy.org/findmod/AMID.html — amidation of C-terminal amino acids,
https://web.expasy.org/findmod/FORM.html — Nε-formylation of lysine,
https://web.expasy.org/findmod/GGLU.html — γ-carboxylation of glutamic acid,
https://web.expasy.org/findmod/HYDR.html — γ-hydroxylation of proline.
Disulfide bonds were taken into account by subtracting two hydrogen atomic masses from the mass of two cysteines. Molecular masses for O-glycosylated polypeptides were calculated only for the aglycone (polypeptide) parts. Table 2 shows good accordance of calculated and measured molecular masses for several Kalium entries.
Table 2.
Comparison of ligand molecular masses measured experimentally and calculated in Kalium.
| UniProt ID | Experimental mass, Da | Kalium calculated mass, Da | Known modifications |
|---|---|---|---|
| Q9U3Z3 | 3569 | 3571.74 | Signal and propeptide cleavage, 4 disulfide bridges, 4 γ-carboxyglutamates, 1 γ-hydroxyproline, C-terminal amidation |
| P0CG45 | 2805.84 | 2807.25 | 3 disulfide bridges, 7 γ-hydroxyprolines |
| Q86QT3 | 4730.8 | 4730.49 | Signal peptide cleavage, 4 disulfide bridges |
| P84704 | 2872.5 | 2873.32 | 2 disulfide bridges |
| P0C166 | 4082.8 | 4081.99 | N-Terminal cyclization of glutamine, C-terminal amidation, 4 disulfide bridges |
Further, the Latin name of every source organism was linked to a valid species entry in the UniProt Taxonomy database (UniProt equivalent of NCBI Taxonomy Browser; http://www.uniprot.org/taxonomy/). Comprehensive activity data were added manually from literature and linked to corresponding references in PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) or DOI. Molecular target nomenclature was adopted as recommended by the International Union of Basic and Clinical Pharmacology (IUPHAR; http://www.guidetopharmacology.org), where it was possible (see “Ligand card”). The data stream and curation process are presented in Fig. 1.
Fig. 1.
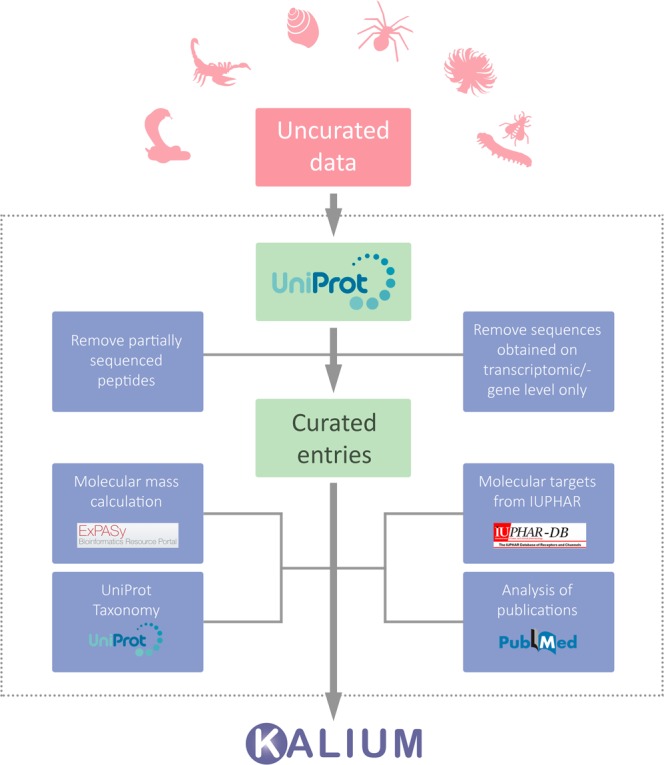
Data sources and curation. Schematic representation of the data stream and curation process in Kalium 2.0.
Implementation
Interface to the Kalium database is centered around the main table with data on K+ channel ligands, initially sorted according to source organism group, organism name and polypeptide family name or common name. The table supports searching, multi-column ordering and filtering, and multi-row selection. BLAST search and sequence alignment using the Clustal Omega program via UniProt web server is implemented, as well as data export for toxins selected by users; all these options are new in Kalium 2.0. Extended information including detailed activity data (the “Ligand card”) is available for each entry as a special popup window.
Kalium is an OpenUI 5 Model-View-Controller web application built upon a Django web framework and SQLite3 database engine. The web interface consists of single dynamically generated HTML5 page with JSON data being fetched from the server asynchronously via AJAX requests. Standard Django web admin interface is used for data access and curation. Modern HTML5-capable browsers (desktop and mobile variants) are supported.
Data Records
Original Kalium 1.0 was assembled as a database of K+ channel toxins from scorpion venom35. Due to database expansion following the addition of K+ channel ligands from other organism sources, the structure of Kalium 2.0 was improved. A copy of Kalium database in CSV format can be accessed at Figshare40.
The main window
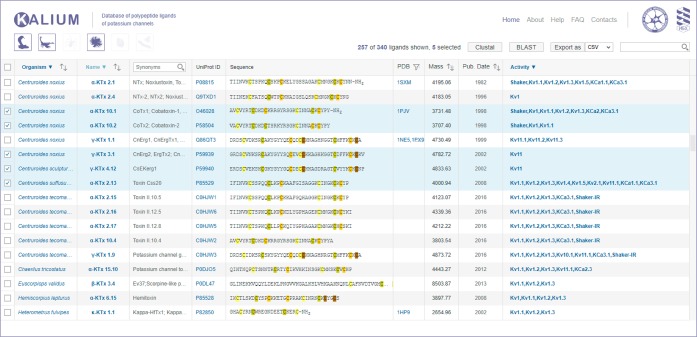
The main window of Kalium is presented by one large general table, in which all data about K+ channel ligands from various sources are assembled (Fig. 2 and Table 3). “Home”, “About”, “Help”, “FAQ”, and “Contacts” located in the top right corner link to pages that contain information about developers and tips. Below those links come buttons “Clustal”, “BLAST”, and “Export as” (a drop-down list of export file format), and a search field. Buttons for source organism selection are located under the Kalium logo in the top left corner. Other control elements of the table are placed in the headers and function to filter information of interest as discussed below. Multiparameter filtering is now an available option in Kalium 2.0.
Fig. 2.
The main window of Kalium 2.0. Top panel consists of the database logo (implementing the home button function), and links to “Home”, “About”, “Help”, “FAQ”, and “Contacts” pages. The second panel contains the organism selection buttons, an indicator of shown and selected entries number, the “Clustal”, “BLAST”, and “Export as” buttons, and a search bar. The main body of the database is presented by a table consisting of fields described in “Data Records”. The figure displays results for the following query: show entries, in which source organisms are snakes, spiders, or sea anemones.
Table 3.
Description of Kalium 2.0 main window general table.
| Table field | Definition |
|---|---|
| Organism | The Latin name of the source organism. |
| Name | The nomenclature name or conventionally used name of polypeptide. |
| Synonyms | Trivial name(s) of polypeptide. |
| UniProt ID | Unique UniProt ID of polypeptide. |
| Sequence | Amino acid sequences of mature polypeptides presented in the one-letter code. “–NH2” indicates amidation of the C-terminal amino acid; “Z” is for the N-terminal pyroglutamic acid; “O” for 4-hydroxyproline; “E” for 4-carboxyglutamic acid; “K” for N6-formyllysine; “T, S” are for O-glycosylated threonine and serine; and “W” is for D-tryptophan. Cysteine residues are marked; different colors indicate the disulfide bond connectivity. |
| PDB | Available PDB ID(s) of polypeptide. |
| Mass | Molecular mass of mature polypeptide calculated taking into account the post-translational modifications. Molecular masses for O-glycosylated polypeptides are marked with the “+” symbol. |
| Publication date | The date when the polypeptide sequence was first published. |
| Activity | The list of all targets on which the polypeptide was ever tested. |
Ligand card
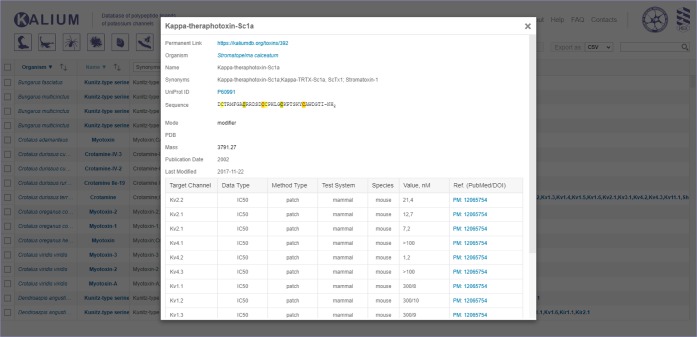
For each polypeptide entry, detailed information is summarized in the “Ligand card” (Fig. 3) available by clicking on polypeptide name in the field “Name” of the general table. As it was implemented in the first Kalium release, all information presented in the general table is duplicated in the Ligand card in an expanded way35. All records of the renewed Ligand card are explained in Tables 3 and 4.
Fig. 3.
Ligand card overview. Kappa-theraphotoxin-Sc1a is taken as an example. All information present in the general table is duplicated here with certain additions (as described in “Ligand card”). Activity data are summarized in a table located to the bottom of the card.
Table 4.
Description of Ligand card records.
| Record | Definition |
|---|---|
| Permanent link | Unique link for the Ligand card which can be used for citation purposes. |
| Raw sequence | Polypeptide precursor sequence (if available). |
| Last modified | The date of the latest modification to the entry. |
| Mode | The mode of ligand action on K+ channels: blocker, blocks ion current by “plugging” the channel pore; modifier, alters channel gating and decreases ion currents by voltage-sensor trapping through binding to extracellular receptor sites; activator, increases ion currents; and undefined. |
| Target channel | K+ channels that were used for toxin activity measurements named according to IUPHAR, except the following: Kxa.b/Kxc.d — heteromeric channel; Shaker, Shab — channels from the fruit fly Drosophila melanogaster; Shaker-IR — Shaker channel with fast N-type inactivation gate removed; KscA-Shaker — chimera of prokaryotic channel KscA from the soil bacterium Streptomyces lividans and Shaker; KvAP — channel from the archaeon Aeropyrum pernix; TSha1 — Shaker-related K+ channel from the trout Oncorhychus mykiss. |
| Data type | The type of data reported: dissociation constant (Kd), inhibition constant (Ki), half-maximal inhibitory concentration (IC50), or half-maximal effective concentration (EC50). |
| Method type | The experimental method applied: radio, radioligand-binding assay; flux, rubidium/thallium efflux assay; patch, electrophysiology using the patch clamp technique; volt, electrophysiology using the voltage clamp technique. |
| Test system | The cell type used for channel expression: insect, Xenopus oocyte, mammalian, or snail (neurons of the mollusk Helix pomatia). |
| Species | The origin organism of the ion channel that was used for measurements. The most common channels belong to fly, rat, mouse, and human organisms. Blank means that the origin of ion channel was not specified in the publication. |
| Value, nM | Numeric value of polypeptide activity (Kd, Ki, IC50 or EC50) presented in nM. These data are collected manually from literature. Values are shown in the following formats: X — Kd, Ki, IC50, or EC50 value in nM; ∼X — approximate Kd, Ki, IC50, or EC50 value in nM; ≥X — ligand had no effect at up to X value; X/Y — means that ligand at concentration X reduced ion current through the channels by Y percent. |
| Ref. (PubMed/DOI) | PubMed ID or DOI of the reference article. |
Those records that duplicate information of the main window general table are described in Table 3.
Export file format
Downloadable text file containing data on Kalium entries is generated in the column-separated (default name is “export.csv”) or tabulation-separated (“export.txt”) format. For multiple selected entries, the file consists of truncated Ligand cards appended one by one. Each truncated Ligand card includes UniProt ID, sequence, list of PDB IDs (if available), molecular mass, and mode of action followed by a table of experimentally determined activity data (if available).
Technical Validation
Database generation process consisted of fetching, filtering and merging manually collected data from the literature and information from the UniProt36. UniProt data validation was not performed, since it is one of the most accurately curated biological resources. The records included in Kalium 2.0 are based on published material in peer-reviewed scientific journals; each specific data value is supported by the original references, so users can evaluate the validity and accuracy of the original source. The overall correctness of the database generation process was verified manually. Mass calculation for mature toxins containing 20 common amino acids and modified residues, was checked against the ExPASy server38,39.
Usage Notes
Kalium 2.0 is freely available for users. Most of the original Kalium 1.0 features were upgraded and new features were implemented, we therefore describe all of them in detail below. Moreover, here we give an example of how Kalium 2.0 can be utilized by researchers with specific needs.
Organism selection buttons
A major new feature of Kalium 2.0 is buttons for organism group selection (Fig. 2). Clicking one or several buttons allows filtering data in the main table according to the source organism groups: snakes, scorpions, spiders, sea anemones, cone snails, and miscellaneous. The “Miscellaneous” group includes K+ channel ligands from fungi, worms, bees, wasps, centipedes, lizards, and humans.
Selecting and manipulating data: Clustal, BLAST, and Export
Check boxes on the left side of the general table permit selection of one or more entries; for all entries selection, users may click once on the column header. Multiple (two or more) entries selection allows performing Clustal alignment request. New features of Kalium 2.0 include an easy BLAST search for multiple sequences and data export for selected polypeptides in a text file.
To submit an alignment request, after entry selection, users need to click the “Clustal” button; the results of Clustal Omega pair/multiple sequence alignment will appear in a new browser tab. Similarly, to submit a BLAST search request, users are required to click the “BLAST” button; the results will appear in separate browser tab for each selected entry. To export data, users are advised to choose the file format (CSV or TXT) in the drop-down list and click the “Export as” button; the resulting file containing data from the selected entries will be generated and sent to the user’s browser.
Organism
The “Organism” header is the control element for filtering and sorting entries by source species names listed according to current biological classification. One click on the column header opens a drop-down menu, where users can choose one or more species to filter the full data set. The Latin names in the table body are linked to the UniProt Taxonomy database ensuring valid classification.
Name
The “Name” header is the control element for filtering and sorting entries by polypeptide families and subfamilies according to current nomenclature. As of February 2019, the filtering option is active for families of scorpion toxins only, since the nomenclature of just these molecules is the most conventional, clear and universally recognized (an updated Tytgat-Possani nomenclature17,41). “Name” enables selecting toxin family from a drop-down menu. Ligand card opens when clicked on toxin name in the table body.
Synonyms
The “Synonyms” header is the control element for searching/filtering trivial names of polypeptides. Many scientists identify certain molecules using trivial names only; therefore their inclusion in Kalium 2.0 is a necessity.
UniProt ID
Click on UniProt ID switches to corresponding UniProt pages.
PDB
The “PDB” header is the control element for filtering entries by PDB ID (if available). Clicking this filter button will show entries with resolved spatial structure only. All PDB IDs are linked to corresponding Protein Data Bank37 pages.
Mass
The “Mass” header is the control element for sorting entries according to molecular mass. One click on this button will sort entries by ascending order of masses, next click — by descending order.
Publication date
The “Publication date” header is the control element for sorting entries according to the date when the sequence was first published.
Activity
The “Activity” header is the control element for filtering and sorting entries by information about activities on different K+ channels. One click on the column header opens a drop-down menu, where users can select one or more channels. The header is used to sort entries according to specific targets. Ligand card can be opened for detailed information by clicking on a channel name.
Ligand card
For user convenience the information of the records “Organism”, “UniProt ID”, “PDB”, and “Ref. (PubMed/DOI)” is linked to corresponding web pages.
Kalium 2.0 application example
Kalium provides convenient tools to analyze the selectivity features of K+ channel ligands. For instance, Kalium may help infer the molecular determinants underlying ligand specificity against particular channel isoforms. Investigators can identify all known polypeptides that were tested against chosen K+ channel isoforms by selecting the appropriate channels in the “Activity” header. The most suitable entries may be selected and analyzed by Clustal or BLAST. As a result, assumptions may be made on potentially important residues42,43 and this information may be further used to produce artificial molecules with enhanced selectivity or affinity. To perform such analysis without using Kalium is difficult, because it is associated with deep literature search. This search has already been performed during data assembly and is central to manual data curation at Kalium.
ISA-Tab metadata file
Acknowledgements
We thank Anton O. Chugunov (Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Moscow, Russia) for discussions concerning the database development. This work was funded by the Molecular and Cell Biology Program of the Russian Academy of Sciences, the Basic Research Program at the National Research University Higher School of Economics and by the Russian Academic Excellence Project ‘5–100’. Literature searches and data collection were performed by V.M.T. supported by the Russian Science Foundation (grant no. 17-74-10172).
Author Contributions
Working group organisers: R.G.E. and A.A.V. A.A.V. provided the idea of the database and upgrades. V.M.T. performed literature searches and data collection. A.I.K. provided the design of the database. N.A.K. performed programing and implementation of the database in the web interface. V.M.T. and A.I.K. performed testing the database efficiency. V.M.T., N.A.K., A.I.K. and A.A.V. wrote the manuscript and all authors contributed to its editing.
Code Availability
Code is available upon request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
ISA-Tab metadata
is available for this paper at 10.1038/s41597-019-0074-x.
References
- 1.Hille, B. Ion Channels of Excitable Membranes. (Sinauer Associates Inc Sunderland, 2001).
- 2.González C, et al. K(+) channels: function-structural overview. Compr. Physiol. 2012;2:2087–2149. doi: 10.1002/cphy.c110047. [DOI] [PubMed] [Google Scholar]
- 3.Doyle DA, et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 4.Heinemann SH, Rettig J, Graack HR, Pongs O. Functional characterization of Kv channel beta-subunits from rat brain. J. Physiol. 1996;493(Pt 3):625–633. doi: 10.1113/jphysiol.1996.sp021409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaczmarek LK, et al. International Union of Basic and Clinical Pharmacology. C. Nomenclature and Properties of Calcium-Activated and Sodium-Activated Potassium Channels. Pharmacol. Rev. 2017;69:1–11. doi: 10.1124/pr.116.012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutman GA, et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol. Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 7.Kubo Y, et al. International Union of Pharmacology. LIV. Nomenclature and molecular relationships of inwardly rectifying potassium channels. Pharmacol. Rev. 2005;57:509–526. doi: 10.1124/pr.57.4.11. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein SAN, et al. International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol. Rev. 2005;57:527–540. doi: 10.1124/pr.57.4.12. [DOI] [PubMed] [Google Scholar]
- 9.Alexander SP, et al. The Concise Guide To Pharmacology 2017/18: Voltage-gated ion channels. Br. J. Pharmacol. 2017;174:S160–S194. doi: 10.1111/bph.13884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuzmenkov AI, Grishin EV, Vassilevski AA. Diversity of Potassium Channel Ligands: Focus on Scorpion. Toxins. Biochem. 2015;80:1764–1799. doi: 10.1134/S0006297915130118. [DOI] [PubMed] [Google Scholar]
- 11.Catterall WA, et al. Voltage-gated ion channels and gating modifier toxins. Toxicon. 2007;49:124–141. doi: 10.1016/j.toxicon.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Norton RS, Chandy KG. Venom-derived peptide inhibitors of voltage-gated potassium channels. Neuropharmacology. 2017;127:124–138. doi: 10.1016/j.neuropharm.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Chhabra S, et al. Kv1.3 channel-blocking immunomodulatory peptides from parasitic worms: implications for autoimmune diseases. FASEB J. 2014;28:3952–3964. doi: 10.1096/fj.14-251967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nobile M, et al. The toxin helothermine affects potassium currents in newborn rat cerebellar granule cells. J. Membr. Biol. 1994;139:49–55. doi: 10.1007/BF00232674. [DOI] [PubMed] [Google Scholar]
- 15.Yang W, et al. Endogenous animal toxin-like human β-defensin 2 inhibits own K(+) channels through interaction with channel extracellular pore region. Cell. Mol. Life Sci. 2015;72:845–853. doi: 10.1007/s00018-014-1715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norton RS. Enhancing the therapeutic potential of peptide toxins. Expert Opin. Drug Discov. 2017;12:611–623. doi: 10.1080/17460441.2017.1317243. [DOI] [PubMed] [Google Scholar]
- 17.Tytgat J, et al. A unified nomenclature for short-chain peptides isolated from scorpion venoms: alpha-KTx molecular subfamilies. Trends Pharmacol. Sci. 1999;20:444–447. doi: 10.1016/S0165-6147(99)01398-X. [DOI] [PubMed] [Google Scholar]
- 18.Mouhat S, Andreotti N, Jouirou B, Sabatier J-M. Animal toxins acting on voltage-gated potassium channels. Curr. Pharm. Des. 2008;14:2503–2518. doi: 10.2174/138161208785777441. [DOI] [PubMed] [Google Scholar]
- 19.Harvey AL, Anderson AJ. Dendrotoxins: snake toxins that block potassium channels and facilitate neurotransmitter release. Pharmacol. Ther. 1985;31:33–55. doi: 10.1016/0163-7258(85)90036-1. [DOI] [PubMed] [Google Scholar]
- 20.Berndt KD, Güntert P, Wüthrich K. Nuclear magnetic resonance solution structure of dendrotoxin K from the venom of Dendroaspis polylepis polylepis. J. Mol. Biol. 1993;234:735–750. doi: 10.1006/jmbi.1993.1623. [DOI] [PubMed] [Google Scholar]
- 21.Peigneur S, et al. Crotamine Pharmacology Revisited: Novel Insights Based on the Inhibition of KV Channels. Mol. Pharmacol. 2012;82:90–96. doi: 10.1124/mol.112.078188. [DOI] [PubMed] [Google Scholar]
- 22.Wang JM, et al. Molecular surface of tarantula toxins interacting with voltage sensors in K(v) channels. J. Gen. Physiol. 2004;123:455–467. doi: 10.1085/jgp.200309005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swartz KJ, MacKinnon R. An inhibitor of the Kv2.1 potassium channel isolated from the venom of a Chilean tarantula. Neuron. 1995;15:941–949. doi: 10.1016/0896-6273(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 24.Swartz KJ, MacKinnon R. Hanatoxin modifies the gating of a voltage-dependent K+ channel through multiple binding sites. Neuron. 1997;18:665–673. doi: 10.1016/S0896-6273(00)80306-2. [DOI] [PubMed] [Google Scholar]
- 25.Liang S. An overview of peptide toxins from the venom of the Chinese bird spider Selenocosmia huwena Wang [=Ornithoctonus huwena (Wang)] Toxicon. 2004;43:575–585. doi: 10.1016/j.toxicon.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Honma T, Shiomi K. Peptide toxins in sea anemones: structural and functional aspects. Mar. Biotechnol. (NY) 2005;8:1–10. doi: 10.1007/s10126-005-5093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terlau H, Olivera BM. Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol. Rev. 2004;84:41–68. doi: 10.1152/physrev.00020.2003. [DOI] [PubMed] [Google Scholar]
- 28.Prashanth JR, Dutertre S, Lewis RJ. Pharmacology of predatory and defensive venom peptides in cone snails. Mol. Biosyst. 2017;13:2453–2465. doi: 10.1039/C7MB00511C. [DOI] [PubMed] [Google Scholar]
- 29.Livett BG, Gayler KR, Khalil Z. Drugs from the sea: conopeptides as potential therapeutics. Curr. Med. Chem. 2004;11:1715–1723. doi: 10.2174/0929867043364928. [DOI] [PubMed] [Google Scholar]
- 30.Bayrhuber M, et al. Conkunitzin-S1 is the first member of a new Kunitz-type neurotoxin family. Structural and functional characterization. J. Biol. Chem. 2005;280:23766–23770. doi: 10.1074/jbc.C500064200. [DOI] [PubMed] [Google Scholar]
- 31.Massilia GR, Schininà ME, Ascenzi P, Polticelli F. Contryphan-Vn: a novel peptide from the venom of the Mediterranean snail Conus ventricosus. Biochem. Biophys. Res. Commun. 2001;288:908–913. doi: 10.1006/bbrc.2001.5833. [DOI] [PubMed] [Google Scholar]
- 32.Lazdunski M. Apamin, a neurotoxin specific for one class of Ca2+ -dependent K+ channels. Cell Calcium. 1983;4:421–428. doi: 10.1016/0143-4160(83)90018-0. [DOI] [PubMed] [Google Scholar]
- 33.Xiang F, et al. Plectasin, First Animal Toxin-Like Fungal Defensin Blocking Potassium Channels through Recognizing Channel Pore Region. Toxins (Basel) 2015;7:34–42. doi: 10.3390/toxins7010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang S, et al. Chemical punch packed in venoms makes centipedes excellent predators. Mol. Cell. Proteomics. 2012;11:640–650. doi: 10.1074/mcp.M112.018853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuzmenkov AI, Krylov NA, Chugunov AO, Grishin EV, Vassilevski AA. Kalium: a database of potassium channel toxins from scorpion venom. Database (Oxford) 2016;2016:baw056. doi: 10.1093/database/baw056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The UniProt Consortium UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2017;45:D158–D169. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berman HM, et al. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkins MR, et al. High-throughput mass spectrometric discovery of protein post-translational modifications. J. Mol. Biol. 1999;289:645–657. doi: 10.1006/jmbi.1999.2794. [DOI] [PubMed] [Google Scholar]
- 39.Bairoch, A. et al. In The Proteomics Protocols Handbook (ed. Walker, J.) Protein identification and analysis tools on the ExPASy server (Humana Press, 2009). [DOI] [PubMed]
- 40.Vassilevski, A., Tabakmakher, V. M., Krylov, N. A., Kuzmenkov, A. I. & Efremov, R. G. Kalium database. figshare10.6084/m9.figshare.6913259.v3 (2019).
- 41.Santibáñez-López CE, Possani LD. Overview of the Knottin scorpion toxin-like peptides in scorpion venoms: Insights on their classification and evolution. Toxicon. 2015;107:317–326. doi: 10.1016/j.toxicon.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 42.Kuzmenkov AI, et al. C-Terminal residues in small potassium channel blockers OdK1 and OSK3 from scorpion venom fine-tune the selectivity. Biochim. Biophys. Acta - Proteins Proteomics. 2017;1865:465–472. doi: 10.1016/j.bbapap.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Kuzmenkov AI, et al. KV1.2 channel-specific blocker from Mesobuthus eupeus scorpion venom: Structural basis of selectivity. Neuropharmacology. 2018;143:228–238. doi: 10.1016/j.neuropharm.2018.09.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Code is available upon request.