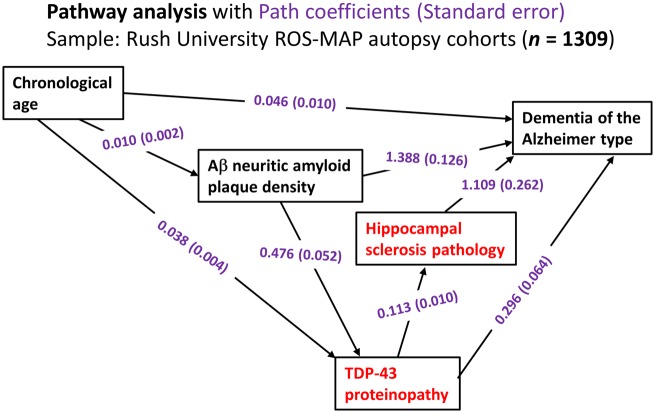
Figure 2.
Statistical analyses on data related to LATE from the Rush University community-based autopsy cohort depicting the results of pathway analyses. Data were analysed from research volunteers (total n = 1309) in two clinical‐pathological studies of ageing from Rush University as described previously (Power et al., 2018). In this sample, the mean age of death was 89.7 years [standard deviation (SD) 6.5 years, range 65–108 years]. These analyses incorporated age, density of amyloid-β neuritic amyloid plaques (to factor in ADNC), TDP-43 proteinopathy, hippocampal sclerosis pathology, and the endpoint of Alzheimer’s-type clinical dementia. The components of the pathway analyses most strongly associated with LATE-NC are shown in red. The numbers are path coefficients with standard error in parentheses (shown in purple). These numbers help to quantify the effects of individual pathways. For instance, the data are compatible with there being two pathways from TDP-43 proteinopathy to dementia, one direct pathway (TDP-43 proteinopathy→dementia) and the other indirect pathway that includes hippocampal sclerosis pathology (TDP-43 proteinopathy→hippocampal sclerosis→dementia): in the statistical model, the TDP-43 proteinopathy is independently associated with both hippocampal sclerosis pathology and clinical dementia status. Further, the data indicate that a subset of TDP-43 proteinopathy is ‘downstream’ of ADNC-type neuritic amyloid plaque pathology. In a practical sense, this means that brains with more neuritic amyloid plaques are more likely to have TDP-43 proteinopathy, with all other known factors being the same. Aβ = amyloid-β.