Figure 2.
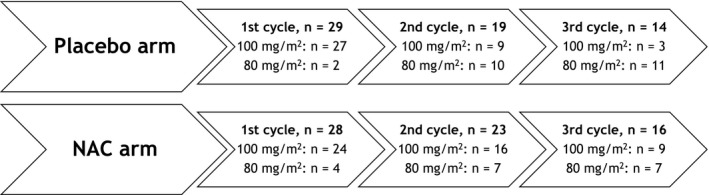
Placebo and NAC arms: number of participants, cisplatin cycles and doses. Considering the placebo arm, from 29 patients that underwent the first cycle, only 19 underwent the second cycle (19/29; 65.5%), and 14 underwent the third cycle (14/29; 48.3%). In the NAC arm, 23 from 28 patients underwent the second cycle (23/28; 82.1%) and 16 underwent the third cycle (16/28; 57.1%). In addition, 10 patients in the placebo arm (10/29; 34.5%) had their dose of cisplatin reduced after the first cycle, vs 6 in the NAC arm (6/28; 21.4%). After the second cycle, 4 patients in the placebo arm had a dose reduction (4/19; 21.1%) vs 4 in the NAC arm (4/23; 17.4%). The reasons for dose reduction or fewer cycles were toxicities and death. The NAC arm underwent more cisplatin cycles and had less dose reduction during treatment
