Abstract
Reactive oxygen species (ROS) promote carcinogenesis by inducing genetic mutations, activating oncogenes, and raising oxidative stress, which all influence cell proliferation, survival, and apoptosis. Cancer cells display redox imbalance due to increased ROS level compared to normal cells. This unique feature in cancer cells may, therefore, be exploited for targeted therapy. Over the past few decades, natural compounds have attracted attention as potential cancer therapies because of their ability to maintain cellular redox homeostasis with minimal toxicity. Preclinical studies show that bioactive dietary polyphenols exert antitumor effects by inducing ROS-mediated cytotoxicity in cancer cells. These bioactive compounds also regulate cell proliferation, survival, and apoptotic and antiapoptotic signalling pathways. In this review, we discuss (i) how ROS is generated and (ii) regulated and (iii) the cell signalling pathways affected by ROS. We also discuss (iv) the various dietary phytochemicals that have been implicated to have cancer therapeutic effects through their ROS-related functions.
1. Introduction
Reactive oxygen species (ROS) are highly reactive metabolic by-products that cause both deleterious and beneficial effects. Cellular ROS act as secondary messengers in signalling cascades that are critical for normal physiological functions such as differentiation and development [1, 2]. However, overproduction of ROS can cause damage to biomolecules such as DNA, lipids, carbohydrates, and proteins [3, 4], leading to loss of cell integrity and subsequently cell pathology (Figure 1). For example, ROS is now recognized to promote tumorigenesis, metastasis, and angiogenesis [5]. But then again, in cancer, excessive accumulation of ROS induces cell death [6]. Studies have shown that cancer cells have increased ROS level compared to normal cells due to high metabolic rate and mitochondrial dysfunction, which render increased susceptibility to oxidative stress [7, 8]. Thus, additional surge in ROS level is likely to cause cancer cells to reach their oxidative stress threshold sooner than normal cells, resulting in oxidative stress-induced cancer cell death [7, 8]. Therefore, it is not surprising that several natural dietary bioactive compounds that cause increased ROS levels have been shown to selectively target cancer cells [9]. For instance, dietary phytochemicals such as polyphenols, flavonoids, and stilbenes have the capacity to inhibit cancer cell proliferation and induce apoptosis and autophagy [10]. While most dietary bioactive compounds possess antioxidant capacity at low doses, high doses induce prooxidant activity that leads to cancer cell death. These compounds also influence mitochondrial functions by altering mitochondrial enzymes, oxidative phosphorylation, and mitochondrial pathways [11]. In this review, we focus on ROS regulation, ROS-mediated signalling pathways, and the contemporary use of dietary phytochemicals for cancer therapy.
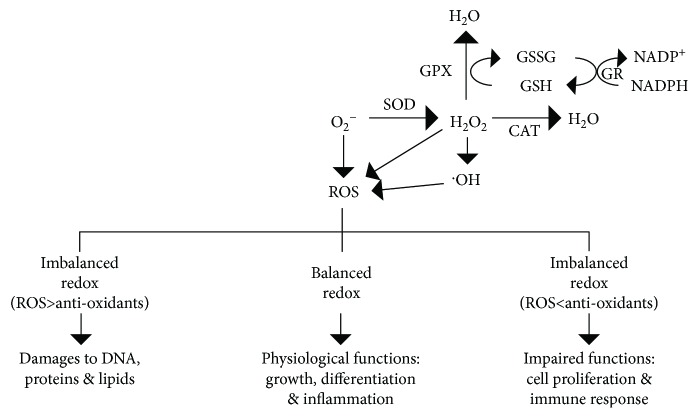
Figure 1.
Intracellular redox homeostasis and imbalance and their effects on cellular functions. SOD: superoxide dismutase; CAT: catalase; OH: hydroxyl radical; GPX: glutathione peroxidase; GSSG: glutathione disulfide; GR: GSSG reductase; GSH: glutathione.
2. ROS Regulation
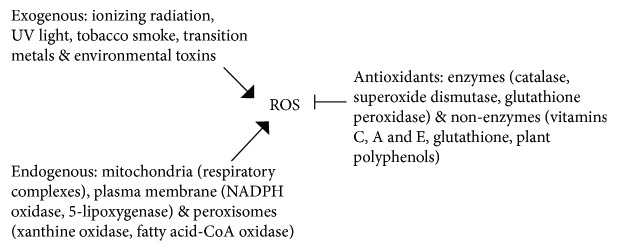
ROS production is affected by both external factors such as tobacco smoke and ionizing radiation and intracellular factors such as the endoplasmic reticulum (ER), mitochondria, and peroxisomes [12] (Figure 2). Endogenous ROS are mainly produced in mitochondria during oxidative phosphorylation. Superoxide anions are generated through the electron transport chain complexes I and III localized in the inner mitochondrial membrane, and superoxide dismutase (SOD) converts superoxide ions into hydrogen peroxide (H2O2), which is subsequently catalyzed by glutathione peroxidase (GPX) to generate H2O. Catalase (CAT) also converts H2O2 to water (Figure 1) [13]. Other intracellular enzymes such as NADPH oxidase, lipoxygenases, and xanthine oxidase are also capable of ROS production [14]. Although intracellular redox homeostasis is well controlled by the enzymatic antioxidants, SOD, GPX, and CAT, it is also regulated by nonenzymatic antioxidants such as ascorbic acid (vitamin C) and glutathione (GSH) [15] (Figure 2).
Figure 2.
Exogenous and endogenous sources of ROS and enzymatic and nonenzymatic antioxidants.
Besides these antioxidants, the transcription factor, nuclear factor erythroid 2- (NFE2-) related factor 2 (Nrf2), also contributes in controlling oxidative stress. Activation of Nrf2 requires inhibition of its negative regulator Keap1, which results in Nrf2 nuclear translocation [16]. This leads to the expression and production of the antioxidant enzymes, CAT, GPX, heme oxygenase-1 (HO-1), and peroxiredoxin (PRX), and maintenance of redox balance [16]. We note, however, that intracellular oxidative stress induces activation of hypoxia-inducible factors (HIFs), resulting in the transcription of genes that promote survival and proliferation of cancer cells [17].
3. ROS in Cancer Signalling Pathways
ROS serve a crucial role in the regulation of a number of cellular processes such as cell proliferation and differentiation and cell death. Therefore, it is critical that a delicate balance in ROS level is maintained. ROS level is regulated by redox homeostasis via ROS elimination through antioxidants. Within the threshold limit of redox homeostasis, a regulated ROS increase could serve as a signal for H2O2-mediated oxidation of protein cysteine residues, triggering specific cellular events such as proliferation [18]. Conversely, disturbance of redox homeostasis in the direction of ROS overload leads to deleterious outcomes such as irreversible oxidative DNA damage that could trigger cell death. It is now known that metabolically transformed and fast-growing cancer cells have higher ROS levels than neighboring normal cells, placing cancer cells at a greater risk of reaching the ROS threshold to induce apoptosis. This infers that promoting further ROS production in cancer cells may be utilized as a strategy to induce cancer cell death.
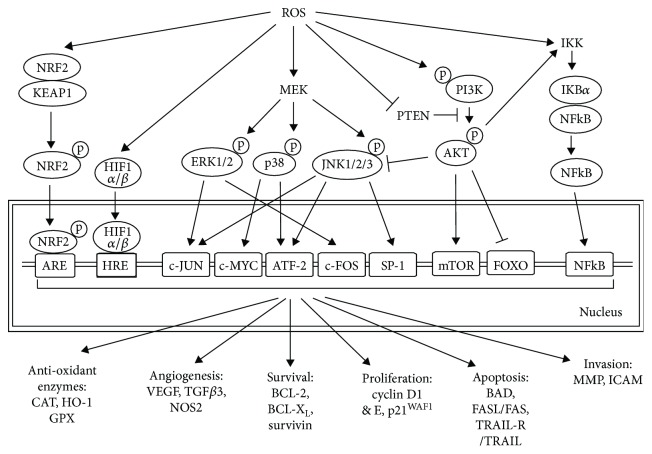
ROS play an important role in tumor initiation, promotion, and progression [19]. At levels below the ROS threshold, ROS activate oncogenes such as Ras and c-Myc [20] and induce p53-mediated DNA repair and survival [21] in cancer cells. At levels above the ROS threshold, ROS trigger apoptotic signals [6]. These cellular processes are controlled by ROS through its regulation of various signalling pathways (Figure 3), including the mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinase (ERK), the phosphoinositide-3-kinase (PI3K)/protein kinase B (AKT), the inhibitor of kappa B (IκB) kinase (IKK)/nuclear factor κB (NFκB), and the protein kinase D (PKD) signalling pathways [22, 23]. For example, ROS-dependent ERK activation controls the expression of proapoptotic genes by phosphorylation of transcription factors [23, 24]. Conversely, ROS-induced JNK activation results in phosphorylation and downregulation of antiapoptotic proteins such as BCL-2 and BCL-XL [25]. In response to ROS, IκB phosphorylation by IKK and subsequently ubiquitination lead to activation and translocation of NFκB into the nucleus to stimulate the expression of antiapoptotic genes [26]. ROS directly activates PI3K subsequently converting phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3) and resulting in transcriptional inhibition of the AKT target genes, glycogen synthase kinase 3 (GSK3), forkhead box O (FOXO), and BCL-2-associated death promoter (BAD) and activation of mammalian target of rapamycin (mTOR1) [27].
Figure 3.
ROS-mediated intracellular cell signalling pathways. The indicated signalling pathways regulate molecules associated with angiogenesis, survival, proliferation, apoptosis, and invasion and the expression of antioxidant enzymes. NRF2: nuclear factor erythroid 2-related factor 2; KEAP1: Kelch-like ECH-associated protein 1; HIF1 α/β: hypoxia inducing factor 1 α/β; HRE: HIF-responsive elements; p38 MAPK: p38 mitogen-activated protein kinase; ERK: extracellular signal-related kinases; MEK: MAPK kinase; JNK; c-Jun N-terminal kinase; PTEN: phosphatase and tensin homolog; PI3K: phosphoinositide-3-kinase; AKT: protein kinase B; IKK: IκB kinase; NFκB: nuclear factor kappa-light-chain-enhancer of activated B cells; FOXO: forkhead box protein O; mTOR1: mechanistic target of rapamycin 1; ATF2: activating transcription factor 2; CAT: catalase; HO-1: heme oxygenase-1; GPX: glutathione peroxidase; VEGF: vascular endothelial growth factor; TGFβ3: transforming growth factor beta 3; NOS2: nitric oxide synthase 2; BCL-2: B-cell lymphoma 2; BCL-XL: B-cell lymphoma-extra large; BAD: BCL2-associated agonist of cell death; TRAIL: TNF-related apoptosis-inducing ligand; MMP: matrix metalloproteinase; ICAM: intercellular adhesion molecule-1.
ROS-mediated apoptosis can be initiated by mitochondrial intrinsic apoptotic signalling or by extrinsic apoptotic signalling through death receptor pathways (Figure 4). Increased production of ROS depolarizes the mitochondrial membrane, releasing cytochrome C from the mitochondria. Cytochrome C induces activation of caspase-9 by promoting nucleotide binding to apoptotic protein-activating factor 1 (APAF-1), which leads to activation of caspase-3 [28]. Antiapoptotic (BCL-2 and BCL-XL) and proapoptotic (BAD, BAK, BAX, BID, and BIM) proteins also contribute to the formation of distinct channels for mitochondrial membrane permeabilization [29]. Elevated ROS levels have also been implicated in the activation of death receptors and in triggering caspase 8-mediated cleavage of caspase 3 [6]. In addition, ROS modulates the TRAIL- and Fas-mediated apoptosis through p53-mediated upregulation of death receptors. p53 regulates such apoptosis by controlling the expression of anti- and proapoptotic (e.g., PUMA and NOXA) proteins [30, 31]. ROS further promotes apoptosis by inducing increased Ca2+-mediated mitochondrial permeability transition pore opening [32].
Figure 4.
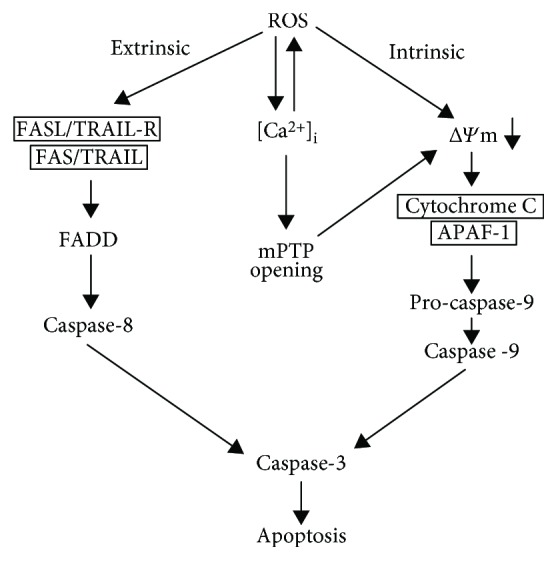
ROS-mediated extrinsic and intrinsic apoptotic pathways. TRAIL: TNF-related apoptosis-inducing ligand; FADD: Fas-associated death domain; [Ca2+]i: intracellular calcium concentration; mPTP: mitochondrial permeability transition pore; ΔΨm: mitochondrial membrane potential.
4. Dietary Polyphenols
There is increasing claim that certain natural bioactive compounds can maintain redox homeostasis and hold promise as anticancer therapeutics due to their biocompatibility, biodegradability, comparatively less toxicity, and reduced side effects. The polyphenol bioactive compounds are secondary metabolites found in plants [33]. The most abundantly occurring plant polyphenols are phenolic acids and flavonoids which account for 30% and 60%, respectively, of dietary polyphenols [33]. Interestingly, they have both antioxidant and prooxidant properties that modulate cell proliferation and apoptotic pathways [34]. Some of the most common bioactive compounds that were suggested to have cancer therapeutic effects through their ROS-related activities are discussed below.
4.1. Quercetin
Quercetin (3,5,7,3′,4′-pentahydroxyflavone) is a flavonoid, present in numerous vegetables and fruits [34, 35]. Quercetin (Qu) displays neuroprotective, chemopreventive, and anticancer activities [36, 37], and these have been attributed to their anti- and prooxidative capacities. Qu efficiently scavenges mitochondrial superoxide anions (O2 −) and subsequently generates semiquinone, Qu radicals, and H2O2 [11, 34, 38]. While, H2O2 is eliminated by peroxidase, semiquinone and Qu radicals alter intracellular ROS metabolism by depleting the intracellular GSH pool in a concentration-dependent manner [39–41] and inhibiting thioredoxin reductase activity [42]. In vitro and in vivo studies (Table 1) show that Qu promotes ROS-induced apoptosis, necrosis, and autophagy [43] at a range of 10-100 μM in a variety of cancers, including glioma [43], osteosarcoma [44], and cervical [45] and breast cancer [46]. Qu induces apoptosis through distinct mechanisms: (i) via the mitochondrial pathway through activation of caspase-3. Qu reduces the mitochondrial membrane potential (MMP), inducing cytochrome C release and subsequent activation of caspase-3. This mechanism was observed in MDA MB-231 breast cancer cells [47], U937 promonocytic leukemia cells [48], HL-60 promyelocytic leukemia cells [49], HepG2 hepatocellular carcinoma cells [50], and oral cancer cells [51]. (ii) Qu alters the expression of the antiapoptotic BCL-2 and BCL-XL and proapoptotic BAX and BAD proteins [47, 48]. Leukemic cells treated with Qu showed upregulation of BAX and increased phosphorylation of BCL-2 [52]. Similar results were observed in osteosarcoma [44] and breast cancer cells [46]. (iii) Qu induces the expression of death receptor- (DR-) 5, enhancing TNF-related apoptosis-inducing ligand- (TRAIL-) induced apoptosis [53–55] either by accumulating death receptors in lipid rafts [56] or inhibiting survivin in the ERK signalling pathway [57]. In addition to its proapoptotic capacity, Qu also promotes cell cycle arrest [58] by modulating p21WAF1, cyclin B, and p27KIP1 in squamous cell carcinoma [59] and breast [60], lung [61], and hepatoma cancer cells [62].
Table 1.
In vivo dosages and mechanistic effects of known natural bioactive compounds.
| Compound | Animals | Cancer model | Dose | Mechanism |
|---|---|---|---|---|
| Quercetin | Male Sprague-Dawley rats | Glioma | 100 mg/kg, every other day for 15 days, i.v | Autophagy and apoptosis [43] |
| Female BALB/c mice | Colon & breast cancer | 100 and 200 mg/kg for 36 days, i.p | Apoptosis [178] | |
| Male BALB/cA nude mice | Prostate cancer | 20 mg/kg for 16 days, i.p | Antiangiogenesis [179] | |
| Female BALB/c/nude mice | Hepatic cancer | 10 mg/kg for 7 days, i.p | Necrosis and antiproliferation [180] | |
| Female NOD.CB17-Prkdcscid/J lineage | Acute myelogenous leukemia | 120 mg/kg, once every 4 days for 21 days, i.p | Apoptosis, autophagy, and cell cycle arrest [181] | |
|
| ||||
| Curcumin | Female BALB/c/nude mice | Colon cancer (multidrug resistance) | 50 mg/kg, 2x/day for 14 days, peritumoral | Reduced expression of MDR1 and survivin [182] |
| Male BALB/c/nude mice | Prostate cancer | 25, 50, and 100 mg/kg, every 2 days for 30 days, abdominal cavity injection | Apoptosis [89] | |
| Female athymic nude mice | Breast cancer | 45 mg/kg, 2x/week for 4 consecutive weeks, i.p | Antiproliferation [183] | |
|
| ||||
| Capsaicin | Female athymic nude mice | Pancreatic cancer | 2.5 & 5 mg/kg, 5x/week, gavage | Activation of JNK and apoptosis [99] |
| Female BALB/c nude mice | Colon cancer | 1 & 3 mg/kg, 3 days once for 40 days, i.p | Apoptosis [95] | |
| Male BNX nu/nu mice | Prostate cancer | 5 mg/kg, 3x/week for 4 weeks, gavage | Antiproliferation and apoptosis [184] | |
| Female BNX nu/nu | Breast cancer | 5 mg/kg, 3x/week for 4 weeks, gavage | Reduced EGFR/HER2 activation and apoptosis [185] | |
|
| ||||
| ECGC | Female C3H/HeJ syngeneic mice | Squamous cell carcinoma | 50 mg/kg, 5 days/week, i.p | Apoptosis [186] |
| NOD/SCID mice | Myeloid leukemia | 10 mM, oral drinking fluid | Antiproliferation [110] | |
| Female BALB/c mice | Bladder cancer | 100 mg/kg for 4 weeks, i.p | Antiproliferation and migration [187] | |
| Male BALB/c/nude mice | Lung cancer | 0.05% in drinking water for 21 days | Angiogenesis [188] | |
| Male BALB/c/nude mice | Adrenal pheochromocytoma | 15 mg/kg, every other day for 15 days, i.p | Apoptosis [189] | |
|
| ||||
| PEITC | Male athymic nude mice | Glioblastoma | 20 μmol/100 μl PBS for 21 days, gavage | Apoptosis [190] |
| Male athymic mice | Prostate cancer | 12 μmol/100 μl PBS for 5 days, oral | Apoptosis [191] | |
| Female BALB/c/nude mice | Lung cancer | 25 mg/kg, 3x/week, i.p | Antiproliferation, reduced cancer stem cells [128] | |
| Female SCID/NOD mice | Breast cancer | 81 mg/kg for 35 days, oral gavage | Apoptosis [192] | |
| Female athymic nude mice | Ovarian cancer | 12 μmol for 42 days, oral gavage | EGFR-AKT pathway inhibition, antiproliferation, and apoptosis [193] | |
|
| ||||
| Piperine | Female BALB/c mice | Mouse 4T1 mammary carcinoma | 2.5 and 5 mg/kg, every 3 days for 3 times, intratumoral | Cell cycle arrest and apoptosis [194] |
| Male nude mice | Prostate cancer | 100 mg/kg/day for 1 month, i.p 10 mg/kg for 1 month, gavage |
Antiproliferation and apoptosis [195] | |
| Male albino Wistar rats | Hepatocellular carcinoma (diethylnitrosamine-induced) | 5 mg/kg, 3x/week for 6 weeks, oral | Apoptosis [138] | |
|
| ||||
| Resveratrol | Male nude mice | Lung cancer | 20 mg/kg, every other day for 25 days, i.p | Reduce metastasis [196] |
| Male BALB/c/nude mice | Bladder cancer | 20 mg/kg/day for 4 weeks, i.p | Decreased VEGF and FGF-2 level, cell cycle arrest, and apoptosis [197] | |
| Female athymic mice | Breast cancer | 25 mg/kg/day for 3 weeks, i.p | Apoptosis [198] | |
| BALB/c/nude mice | Pancreatic cancer | 20, 40, and 60 mg/kg, 5 days/week for 6 weeks, gavage | Inhibition of FOXO transcription factors and apoptosis [199] | |
| Male athymic nude mice | Prostate cancer | 50 mg/kg, every other day for 2 weeks, gavage | Antiproliferation [200] | |
i.p: intraperitoneal; i.v: intravenous.
4.2. Curcumin
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is the principal polyphenol derived from turmeric (Curcuma longa). Various pharmacological activities have been attributed to curcumin, including its anti-inflammatory and anticarcinogenic properties which are triggered at 25 μM [63]. Its anticancer effect is currently being evaluated in clinical trials for a variety of cancers [64–66] (Table 2). In normal cells, curcumin acts as a potent antioxidant. It scavenges hydroxyl radicals, superoxide, nitric oxide, H2O2, and peroxynitrite [11, 67–69] and modulates the expression of SOD, HO-1, and GPX through an indirect mechanism [11, 70–72]. In contrast, curcumin's anticancer properties rely on its prooxidative capacity to induce apoptosis, likely via the mitochondria-mediated pathway [73–75]. Curcumin oxidizes thiols in the mitochondrial membrane, leading to mitochondrial permeability transition pore (mPTP) opening, mitochondrial swelling, mitochondrial depolarization, and inhibition of ATP synthesis, resulting in apoptosis [76]. Evidence shows that curcumin increases ROS levels, including superoxides, hydroxy radicals, and H2O2 [77–79]. Indeed, in human hepatoma cells, curcumin causes cell death by ROS-induced mitochondrial DNA damage and impairment of OXPHOS [80, 81]. Curcumin also activates TRAIL-induced apoptosis by ROS-mediated upregulation of DR5 in renal cancer cells and colon cancer cells [82, 83]. Curcumin further induces autophagy in colon cancer cells through ROS-dependent activation of the ERK1/2 and the p38 MAPK pathway [84]. In glioblastoma [85] and liver cancer [86], curcumin decreases cancer stem cell viability and proliferation by ROS-mediated inhibition of NFκB and signal transducer and activator of transcription 3 (STAT3). As with Qu, curcumin promotes cancer cell apoptosis by upregulating proapoptotic proteins (BAX, BIM, BAK, and NOXA) [87, 88] and downregulating antiapoptotic proteins (BCL-2 and BCL-XL) [89, 90]. In addition, curcumin can impede tumor angiogenesis by downregulating the expression of the vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) [91, 92].
Table 2.
Clinical trials of natural phytochemicals.
| Bioactive compounds (Clinicaltrials.gov identifier) |
Disease condition | Phase | Dosage | Study goal |
|---|---|---|---|---|
|
Quercetin
(NCT03476330) |
Squamous cell carcinoma | II | 4 g/day | Efficacy in reducing buccal micronuclei in patients with Fanconi anemia |
|
Curcumin
(NCT03769766) |
Prostate cancer | III | 500 mg, 2x/day | Effect on prostate cancer progression |
| (NCT00094445) | Pancreatic cancer | II | 8 g/day | Effect in pancreatic cancer growth and the safety of treatment |
| (NCT01246973) | Radiation dermatitis | III | 500 mg, 3x/day | Effect on dermatitis caused by radiation therapy in breast cancer patients |
| With piperine (NCT02598726) |
Neoplasms | I | A dose escalation study | Optimal biological dose in cancer patients |
|
Capsaicin
(NCT02037464) |
Prostate cancer | II | 2 capsules/day for 6 months | Expression of Ki67 and p27 in a posttreatment biopsy |
| (NCT00003610) | Head & neck cancer, mucositis | III | 4 lozenges/day up to 2 weeks after radiation therapy | Efficacy of lozenges in patients with mucositis caused by radiation therapy |
| Patch (Qutenza) (NCT03317613) | Cancer | II | Qutenza (8% capsaicin patch) for every 3 months | Efficacy in peripheric neuropathic pain in cancer patients |
|
EGCG
(NCT02891538) |
Colon cancer | Early I | 450 mg, 2x/day | Chemopreventive effects |
| (NCT01317953) | Small cell lung carcinoma | I | 2 × 450 mg/day to 5 × 450 mg/day | Side effects and best dose |
|
PEITC
(NCT00691132) |
Lung cancer | II | 4x/day for 5 days in week 4 | Effect in preventing lung cancer in smokers |
| (NCT01790204) | Oral cancer | I & II | Effect on oral cells with mutant p53 | |
| Nutri-PEITC jelly (NCT03034603) |
Head & neck neoplasms | 200 mg/day, 5 days/week for 3 months | Safety and efficacy | |
|
Resveratrol
(NCT00256334) |
Colon cancer | I | 20 mg/day | Modulation of Wnt signalling in vivo |
| (NCT01476592) | Neuroendocrine tumor | 5 g/day | Effect on Notch-1 signalling | |
|
SRT501
(NCT00920803) |
Colorectal cancer | I | 5 g/day | Safety and tolerability |
4.3. Capsaicin
Capsaicin (trans-8-methyl-N-vanillyl-6-nonenamide), the major component of Capsicum [93], has been implicated to have anticarcinogenic properties [94–96]. However, the mechanisms by which capsaicin induces cancer cell death are still unclear. The proposed anticancer mechanisms of capsaicin include promotion of ROS accumulation, mitochondria-mediated apoptosis, cell cycle arrest, and impairment of endoplasmic reticulum (ER) calcium homeostasis [97]. Capsaicin induces a rapid rise of ROS level followed by a disruption of mitochondrial membrane potential and subsequent activation of downstream caspase-3 in human colon cancer [98], pancreatic cancer [99], glioma [100], and prostate cancer [101]. In transformed T-cells, capsaicin inhibits the plasma membrane NADH-oxidoreductase (PMOR) electron transport chain, causing an increase in ROS level and subsequent disruption of the mitochondrial membrane potential [102]. Capsaicin at 150 μM also blocks complexes I and III of the respiratory chain and decreases SOD activity in pancreatic cancer [103]. Interestingly, binding of capsaicin to the transient receptor potential vanilloid type 1 (TRPV1) results in an increase in intracellular calcium level and activation of the apoptotic pathway [104–106]. Besides its proapoptotic effects, capsaicin can also induce cell cycle arrest through inhibition of the cyclin-dependent kinases, Cdk2, Cdk4, and Cdk6 [107, 108].
4.4. Epigallocatechin-3-Gallate (EGCG)
Epigallocatechin-3-gallate ((2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3-yl 3,4,5-trihydroxybenzoate) is a prominent catechin polyphenol in green tea. EGCG has dual antioxidant and prooxidant roles. It produces ROS by autooxidation [109] and its ability to modulate ROS level accounts for its chemopreventive property. EGCG induces apoptosis in various cancer cell types, including myeloid leukemia cells [110], human lymphoblastoid B cells [111], and hepatocarcinoma cells [112]. In pancreatic carcinoma [113] and lung cancer cells [114], EGCG-induced apoptosis occurs through inhibition of the PI3K/AKT signalling pathway. EGCG also decreases the mitochondrial membrane potential, increasing the intracellular free Ca2+ level and causing activation of the intrinsic apoptotic pathway. EGCG further decreases the expression of the antiapoptotic BCL-2, BCL-XL, xIAP, and cIAP and increases the expression of the proapoptotic BAD, BAX, and FAS/CD95 [115]. In pancreatic [116] and bladder cancer cells [117], EGCG also induces G0/G1 cell cycle arrest through regulation of cyclin D1, Cdk4, Cdk6, p21WAF1, and p27KIP1 via the ERK, IKK, and PI3K signalling pathways. A combination of EGCG (10 μM) and curcumin (10 μM) inhibits breast cancer stem cell growth by inactivating the NFκB-STAT3 pathway [118].
4.5. PEITC and BITC
Phenethyl isothiocyanate (PEITC) and benzyl isothiocyanate (BITC) are abundant in cruciferous vegetables that have been implicated to have anticancer properties [119–122]. Epidemiological studies show that increased intake of dietary isothiocyanates (ITC) reduces cancer risk [123] and increases cancer patient survival [124]. Both PEITC and BITC induce ROS production in many cancer cells [125–127]. IC50 value of PEITC is at the range of 3-14 μM in various human cancer cells [128]. PEITC increases ROS level by decreasing intracellular GSH level, leading to mitochondrial dysfunction as observed in ovarian [126, 129] and non-small-cell lung cancer [128] cells but not in normal cells. PEITC-induced ROS production correlates with inhibition of complex III activity, inhibition of OXPHOS, and ATP depletion in prostate cancer [125]. PEITC also inhibits HO-1 and subsequently induces the ROS-mediated mitochondrial apoptotic pathway, which was noted in human chronic myeloid leukemia [130]. Conversely, BITC causes oxidative stress in pancreatic [131], glioma [122], and prostate cancer [132] cells by depleting SOD and GSH, which is accompanied by the induction of caspase-mediated apoptosis [121, 133]. BITC also activates the ERK/JNK/p38MAPK pathway in pancreatic cancer [134]. Both PEITC and BITC induce G2/M cell cycle arrest by downregulating cyclin B1, Cdc2, and Cdc25C [135, 136].
4.6. Piperine
Piperine ([5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]piperidine) is the most abundant natural alkaloid found in long pepper (Piper longum L.). Recently, it was determined to be a promising anticancer compound [137]. Piperine suppresses tumor growth in vitro and in vivo by modulating the ROS-induced oxidative stress response pathway, cell cycle arrest, and ER stress. In hepatocellular carcinoma, piperine treatment initiates ROS-induced mitochondria-mediated apoptosis by inhibiting catalase activity [138]. In human oral squamous cells exposed to high concentrations of piperine, ROS elevation is associated with mitochondrial depolarization and activation of caspase-mediated apoptosis. Piperine also induces nuclear condensation and cell cycle arrest in these cells [139].
4.7. Resveratrol
Resveratrol (3,4′,5-trihydroxystilbene), a polyphenol that is found in grapes and berries, effectively prevents tumor initiation and progression by stimulating apoptosis at 10 to 100 μM [140] in prostate [141] and neuroblastoma cells [142]. Resveratrol has been shown to promote apoptosis by activating p53, ROS-dependent caspases, and death receptors for TRAIL and FasL [143]. Resveratrol-mediated apoptosis is mainly associated with the inhibition of the PI3K/AKT, MAPK, and NFκB pathways [144] and STAT3 [145]. Moreover, resveratrol suppresses the expression of antiapoptotic proteins such as survivin, xIAP, and BCL-XL and increases BAX/caspase-3-associated apoptosis [146]. Resveratrol further binds to F1-ATPase, inhibiting mitochondrial ATP synthesis [147, 148]. It triggers cell cycle arrest by upregulating p21WAF1 and p27KIP1 and downregulating cyclins D1, D2, and E and Cdks 2, 4, and 6 [149, 150].
4.8. Others
Peanuts, tomatoes, and carrots are rich in p-Coumaric acid (p-CoA), an isomer of cinnamic acid [151]. In colon cancer cells, p-CoA triggers apoptosis by increasing ROS generation and mitochondrial depolarization, resulting in p53-mediated upregulation of BAX and downregulation of BCL-2 [151, 152]. In addition, p-CoA treatment of these cells in vitro and in vivo induces apoptosis mediated by the unfolded protein response [153].
The naturally occurring quinone compounds have potent cytotoxicity against cancer cells. In lung adenocarcinoma cells, 2-methoxy-1,4-naphthoquinone (MNQ) and 8-hydroxy-2-methoxy-1,4-naphthoquinone (HMNQ) elicit ROS production and induce apoptosis via the JNK/p38 MAPK pathway [154–156].
Naringenin, a citrus flavonoid, triggers ROS-induced apoptosis and stimulates p38MAPK-mediated caspase activation [157, 158].
Gallic acid (3,4,5-trihydroxy-benzoic acid; GA), which is widely present in grapes and red wine, inhibits lung cancer cell growth by increasing ROS level and depleting GSH [159]. In prostate cancer cells, autooxidation of GA produces H2O2 and O2 −, leading to mitochondria-dependent apoptosis [160]. GA also induces apoptosis via ROS-dependent activation of the ATM/p53 [161] and JNK pathways [162].
5. Limitations
Poor bioavailability is a major obstacle for natural bioactive compounds, especially for Qu, curcumin, and resveratrol, which are associated with poor absorption and fast metabolism in the liver and intestine. Pharmacokinetic profile analysis of Qu revealed that about 93% of the compound is metabolised after oral administration (10 mg/kg) in male Sprague-Dawley rats [163]. On the other hand, people taking high oral doses (10 or 12 g) of curcumin attained limited availability of this compound in the plasma and other tissues [164]. Similarly, oral bioavailability of resveratrol is low at less than 1% [165]. Thus, the cytotoxic concentration of these compounds appears to be difficult to achieve by oral administration in cancer patients [166]. Several strategies have been proposed to overcome the problem of low oral bioavailability. One approach is to use a combination of phytochemicals. For example, a combination of piperine and curcumin [167] (in rats: 20 mg/kg piperine + 2 g/kg curcumin; in humans: 20 mg piperine + 2 g curcumin) or piperine and resveratrol [168] (in mice: 10 mg/kg piperine + 100 mg/kg resveratrol) showed increased bioavailability of curcumin and resveratrol, respectively. Other promising approaches include the use of novel formulations, synthetic analogues, prodrugs, and different drug delivery systems (e.g., via liposomes, phospholipid complexes, micelles, and nanoparticles). These methods could increase bioavailability as well as solubility and/or metabolic stability [169, 170]. Some studies have also shown that natural bioactive compounds may promote carcinogenesis by inducing ROS-mediated chromosome aberrations and DNA damage [80, 171, 172]. For example, an in vivo study showed that curcumin promotes lung cancer [173] and topical application of capsaicin causes skin cancer in mice [174], suggesting that these natural compounds must be carefully assessed for safety prior to clinical application.
As dietary phytochemicals lack mechanistic selectivity, these natural compounds display a variety of effects in different cancer cell types and thus the discrepancies in results among separate studies. Other possible reasons for divergent findings in different studies include changes or differences in (i) stability of the bioactive compounds in cell culture medium, for example, stability of Qu decreases at pH 7 or 8 [175]; (ii) release of bioactive compounds under different conditions, for example, the maximum release of curcumin occurs in phosphate buffered saline at pH 6.4 [176]; (iii) sensitivity of different cell types to bioactive compounds; (iv) cellular permeability of bioactive compounds; (v) presence or contamination by metal ions [177]; (vi) number of hydroxyl groups present in a molecule [177]; and (vii) in vivo biodistribution.
6. Conclusion
Natural phytochemicals have been associated with anticancer properties through their ability to modulate oxidative stress, cell cycle regulators, and proapoptotic, antiapoptotic, and survival signalling pathways. In preclinical and clinical trials, bioactive compounds show a promising and wide therapeutic window against various malignancies, including glioblastoma and breast, colon, and prostate cancers where phytochemical-induced cancer cell death was observed. However, certain attributes such as poor solubility and bioavailability of these bioactive compounds limit their clinical application. Thus, further studies are required to identify ways for effective biological delivery of these compounds in different cancer cell types. It is also critical that detailed studies are conducted in large cohorts to establish the pharmacokinetic profile of these compounds alone and in combination with other chemotherapeutic agents to determine dosage, tissue targets, and toxicity. Indeed, natural phytochemicals may serve as future therapy for specific types of cancer.
Acknowledgments
This work was supported in part by grants from the CIHR (MOP-123400) and NSERC (RGPIN/312985-2011) to KYL and an Alberta Cancer Foundation graduate studentship to SN.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
SN wrote the draft. JR and KYL revised the manuscript.
References
- 1.Sena L. A., Chandel N. S. Physiological roles of mitochondrial reactive oxygen species. Molecular Cell. 2012;48(2):158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Covarrubias L., Hernandez-Garcia D., Schnabel D., Salas-Vidal E., Castro-Obregon S. Function of reactive oxygen species during animal development: passive or active? Developmental Biology. 2008;320(1):1–11. doi: 10.1016/j.ydbio.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 3.Martin K. R., Barrett J. C. Reactive oxygen species as double-edged swords in cellular processes: low-dose cell signaling versus high-dose toxicity. Human & Experimental Toxicology. 2002;21(2):71–75. doi: 10.1191/0960327102ht213oa. [DOI] [PubMed] [Google Scholar]
- 4.Bellance N., Lestienne P., Rossignol R. Mitochondria: from bioenergetics to the metabolic regulation of carcinogenesis. Frontiers in Bioscience. 2009;14:4015–4034. doi: 10.2741/3509. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan L. B., Chandel N. S. Mitochondrial reactive oxygen species and cancer. Cancer & Metabolism. 2014;2(1) doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redza-Dutordoir M., Averill-Bates D. A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochimica et Biophysica Acta. 2016;1863(12):2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nature Reviews Drug Discovery. 2009;8(7):579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 8.NavaneethaKrishnan S., Rosales J. L., Lee K. Y. Targeting Cdk5 for killing of breast cancer cells via perturbation of redox homeostasis. Oncoscience. 2018;5(5-6):152–154. doi: 10.18632/oncoscience.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mileo A. M., Miccadei S. Polyphenols as modulator of oxidative stress in cancer disease: new therapeutic strategies. Oxidative Medicine and Cellular Longevity. 2016;2016:17. doi: 10.1155/2016/6475624.6475624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Vallinas M., Gonzalez-Castejon M., Rodriguez-Casado A., Ramirez de Molina A. Dietary phytochemicals in cancer prevention and therapy: a complementary approach with promising perspectives. Nutrition Reviews. 2013;71(9):585–599. doi: 10.1111/nure.12051. [DOI] [PubMed] [Google Scholar]
- 11.Gibellini L., Bianchini E., De Biasi S., Nasi M., Cossarizza A., Pinti M. Natural compounds modulating mitochondrial functions. Evidence-Based Complementary and Alternative Medicine. 2015;2015:13. doi: 10.1155/2015/527209.527209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phaniendra A., Jestadi D. B., Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian Journal of Clinical Biochemistry. 2015;30(1):11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabharwal S. S., Schumacker P. T. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nature Reviews Cancer. 2014;14(11):709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown D. I., Griendling K. K. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circulation Research. 2015;116(3):531–549. doi: 10.1161/CIRCRESAHA.116.303584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mironczuk-Chodakowska I., Witkowska A. M., Zujko M. E. Endogenous non-enzymatic antioxidants in the human body. Advances in Medical Sciences. 2018;63(1):68–78. doi: 10.1016/j.advms.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 16.D'Autreaux B., Toledano M. B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nature Reviews Molecular Cell Biology. 2007;8(10):813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 17.Galanis A., Pappa A., Giannakakis A., Lanitis E., Dangaj D., Sandaltzopoulos R. Reactive oxygen species and HIF-1 signalling in cancer. Cancer Letters. 2008;266(1):12–20. doi: 10.1016/j.canlet.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 18.Schieber M., Chandel N. S. ROS function in redox signaling and oxidative stress. Current Biology. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liou G. Y., Storz P. Reactive oxygen species in cancer. Free Radical Research. 2010;44(5):479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumari S., Badana A. K., G M. M., G S., Malla R. R. Reactive oxygen species: a key constituent in cancer survival. Biomarker Insights. 2018;13:p. 117727191875539. doi: 10.1177/1177271918755391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu B., Chen Y., St. Clair D. K. ROS and p53: a versatile partnership. Free Radical Biology & Medicine. 2008;44(8):1529–1535. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moloney J. N., Cotter T. G. ROS signalling in the biology of cancer. Seminars in Cell & Developmental Biology. 2018;80:50–64. doi: 10.1016/j.semcdb.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J., Wang X., Vikash V., et al. ROS and ROS-mediated cellular signaling. Oxidative Medicine and Cellular Longevity. 2016;2016:18. doi: 10.1155/2016/4350965.4350965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Z., Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006;58(11):621–631. doi: 10.1080/15216540600957438. [DOI] [PubMed] [Google Scholar]
- 25.Dhanasekaran D. N., Reddy E. P. JNK signaling in apoptosis. Oncogene. 2008;27(48):6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mincheva-Tasheva S., Soler R. M. NF-κB Signaling Pathways. The Neuroscientist. 2013;19:175–194. doi: 10.1177/1073858412444007. [DOI] [PubMed] [Google Scholar]
- 27.Manning B. D., Cantley L. C. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NavaneethaKrishnan S., Rosales J. L., Lee K. Y. Loss of Cdk5 in breast cancer cells promotes ROS-mediated cell death through dysregulation of the mitochondrial permeability transition pore. Oncogene. 2018;37(13):1788–1804. doi: 10.1038/s41388-017-0103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Youle R. J., Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature reviews Molecular Cell Biology. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 30.Lee D. H., Rhee J. G., Lee Y. J. Reactive oxygen species up-regulate p53 and Puma; a possible mechanism for apoptosis during combined treatment with TRAIL and wogonin. British Journal of Pharmacology. 2009;157(7):1189–1202. doi: 10.1111/j.1476-5381.2009.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida K., Miki Y. The cell death machinery governed by the p53 tumor suppressor in response to DNA damage. Cancer Science. 2010;101(4):831–835. doi: 10.1111/j.1349-7006.2009.01488.x. [DOI] [PubMed] [Google Scholar]
- 32.Hempel N., Trebak M. Crosstalk between calcium and reactive oxygen species signaling in cancer. Cell Calcium. 2017;63:70–96. doi: 10.1016/j.ceca.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. The Journal of Nutritional Biochemistry. 2007;18(7):427–442. doi: 10.1016/j.jnutbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Gibellini L., Pinti M., Nasi M., et al. Interfering with ROS metabolism in cancer cells: the potential role of quercetin. Cancers. 2010;2(2):1288–1311. doi: 10.3390/cancers2021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong C. H., Joo S. H. Downregulation of reactive oxygen species in apoptosis. Journal of Cancer Prevention. 2016;21(1):13–20. doi: 10.15430/JCP.2016.21.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dajas F. Life or death: neuroprotective and anticancer effects of quercetin. Journal of Ethnopharmacology. 2012;143(2):383–396. doi: 10.1016/j.jep.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Chen C., Zhou J., Ji C. Quercetin: a potential drug to reverse multidrug resistance. Life Sciences. 2010;87(11-12):333–338. doi: 10.1016/j.lfs.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Metodiewa D., Jaiswal A. K., Cenas N., Dickancaite E., Segura-Aguilar J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radical Biology & Medicine. 1999;26(1-2):107–116. doi: 10.1016/S0891-5849(98)00167-1. [DOI] [PubMed] [Google Scholar]
- 39.Kachadourian R., Day B. J. Flavonoid-induced glutathione depletion: potential implications for cancer treatment. Free radical Biology & Medicine. 2006;41(1):65–76. doi: 10.1016/j.freeradbiomed.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferraresi R., Troiano L., Roat E., et al. Essential requirement of reduced glutathione (GSH) for the anti-oxidant effect of the flavonoid quercetin. Free Radical Research. 2005;39(11):1249–1258. doi: 10.1080/10715760500306935. [DOI] [PubMed] [Google Scholar]
- 41.Ramos A. M., Aller P. Quercetin decreases intracellular GSH content and potentiates the apoptotic action of the antileukemic drug arsenic trioxide in human leukemia cell lines. Biochemical Pharmacology. 2008;75(10):1912–1923. doi: 10.1016/j.bcp.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Lu J., Papp L. V., Fang J., Rodriguez-Nieto S., Zhivotovsky B., Holmgren A. Inhibition of mammalian thioredoxin reductase by some flavonoids: implications for myricetin and quercetin anticancer activity. Cancer Research. 2006;66(8):4410–4418. doi: 10.1158/0008-5472.CAN-05-3310. [DOI] [PubMed] [Google Scholar]
- 43.Bi Y., Shen C., Li C., et al. Inhibition of autophagy induced by quercetin at a late stage enhances cytotoxic effects on glioma cells. Tumor Biology. 2016;37(3):3549–3560. doi: 10.1007/s13277-015-4125-4. [DOI] [PubMed] [Google Scholar]
- 44.Xie X., Yin J., Jia Q., et al. Quercetin induces apoptosis in the methotrexate-resistant osteosarcoma cell line U2-OS/MTX300 via mitochondrial dysfunction and dephosphorylation of Akt. Oncology Reports. 2011;26(3):687–693. doi: 10.3892/or.2011.1328. [DOI] [PubMed] [Google Scholar]
- 45.Vidya Priyadarsini R., Senthil Murugan R., Maitreyi S., Ramalingam K., Karunagaran D., Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. European Journal of Pharmacology. 2010;649(1-3):84–91. doi: 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 46.Choi E. J., Bae S. M., Ahn W. S. Antiproliferative effects of quercetin through cell cycle arrest and apoptosis in human breast cancer MDA-MB-453 cells. Archives of Pharmacal Research. 2008;31(10):1281–1285. doi: 10.1007/s12272-001-2107-0. [DOI] [PubMed] [Google Scholar]
- 47.Chien S. Y., Wu Y. C., Chung J. G., et al. Quercetin-induced apoptosis acts through mitochondrial- and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Human & Experimental Toxicology. 2009;28(8):493–503. doi: 10.1177/0960327109107002. [DOI] [PubMed] [Google Scholar]
- 48.Lugli E., Troiano L., Ferraresi R., et al. Characterization of cells with different mitochondrial membrane potential during apoptosis. Cytometry Part A. 2005;68(1):28–35. doi: 10.1002/cyto.a.20188. [DOI] [PubMed] [Google Scholar]
- 49.Wang I. K., Lin-Shiau S. Y., Lin J. K. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. European journal of cancer. 1999;35(10):1517–1525. doi: 10.1016/S0959-8049(99)00168-9. [DOI] [PubMed] [Google Scholar]
- 50.Granado-Serrano A. B., Martin M. A., Bravo L., Goya L., Ramos S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2) The Journal of Nutrition. 2006;136(11):2715–2721. doi: 10.1093/jn/136.11.2715. [DOI] [PubMed] [Google Scholar]
- 51.Kang J. W., Kim J. H., Song K., Kim S. H., Yoon J. H., Kim K. S. Kaempferol and quercetin, components of Ginkgo biloba extract (EGb 761), induce caspase-3-dependent apoptosis in oral cavity cancer cells. Phytotherapy Research. 2010;24(Suppl 1):S77–S82. doi: 10.1002/ptr.2913. [DOI] [PubMed] [Google Scholar]
- 52.Duraj J., Zazrivcova K., Bodo J., Sulikova M., Sedlak J. Flavonoid quercetin, but not apigenin or luteolin, induced apoptosis in human myeloid leukemia cells and their resistant variants. Neoplasma. 2005;52(4):273–279. [PubMed] [Google Scholar]
- 53.Jung Y. H., Heo J., Lee Y. J., Kwon T. K., Kim Y. H. Quercetin enhances TRAIL-induced apoptosis in prostate cancer cells via increased protein stability of death receptor 5. Life Sciences. 2010;86(9-10):351–357. doi: 10.1016/j.lfs.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J. Y., Kim E. H., Park S. S., Lim J. H., Kwon T. K., Choi K. S. Quercetin sensitizes human hepatoma cells to TRAIL-induced apoptosis via Sp1-mediated DR5 up-regulation and proteasome-mediated c-FLIPS down-regulation. Journal of Cellular Biochemistry. 2008;105(6):1386–1398. doi: 10.1002/jcb.21958. [DOI] [PubMed] [Google Scholar]
- 55.Chen W., Wang X., Zhuang J., Zhang L., Lin Y. Induction of death receptor 5 and suppression of survivin contribute to sensitization of TRAIL-induced cytotoxicity by quercetin in non-small cell lung cancer cells. Carcinogenesis. 2007;28(10):2114–2121. doi: 10.1093/carcin/bgm133. [DOI] [PubMed] [Google Scholar]
- 56.Psahoulia F. H., Drosopoulos K. G., Doubravska L., Andera L., Pintzas A. Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Molecular Cancer Therapeutics. 2007;6(9):2591–2599. doi: 10.1158/1535-7163.MCT-07-0001. [DOI] [PubMed] [Google Scholar]
- 57.Kim Y. H., Lee D. H., Jeong J. H., Guo Z. S., Lee Y. J. Quercetin augments TRAIL-induced apoptotic death: involvement of the ERK signal transduction pathway. Biochemical Pharmacology. 2008;75(10):1946–1958. doi: 10.1016/j.bcp.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gibellini L., Pinti M., Nasi M., et al. Quercetin and cancer chemoprevention. Evidence-Based Complementary and Alternative Medicine. 2011;2011:15. doi: 10.1093/ecam/neq053.591356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Q., Zhao X. H., Wang Z. J. Cytotoxicity of flavones and flavonols to a human esophageal squamous cell carcinoma cell line (KYSE-510) by induction of G2/M arrest and apoptosis. Toxicology in Vitro. 2009;23(5):797–807. doi: 10.1016/j.tiv.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 60.Jeong J. H., An J. Y., Kwon Y. T., Rhee J. G., Lee Y. J. Effects of low dose quercetin: cancer cell-specific inhibition of cell cycle progression. Journal of Cellular Biochemistry. 2009;106(1):73–82. doi: 10.1002/jcb.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang J. H., Hsia T. C., Kuo H. M., et al. Inhibition of lung cancer cell growth by quercetin glucuronides via G2/M arrest and induction of apoptosis. Drug Metabolism and Disposition. 2006;34(2):296–304. doi: 10.1124/dmd.105.005280. [DOI] [PubMed] [Google Scholar]
- 62.Mu C., Jia P., Yan Z., Liu X., Li X., Liu H. Quercetin induces cell cycle G1 arrest through elevating Cdk inhibitors p21 and p27 in human hepatoma cell line (HepG2) Methods and Findings in Experimental and Clinical Pharmacology. 2007;29(3):179–183. doi: 10.1358/mf.2007.29.3.1092095. [DOI] [PubMed] [Google Scholar]
- 63.Aggarwal B. B., Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends in Pharmacological Sciences. 2009;30(2):85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Strimpakos A. S., Sharma R. A. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxidants & Redox Signaling. 2008;10(3):511–546. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 65.Aggarwal B. B., Kumar A., Bharti A. C. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Research. 2003;23(1A):363–398. [PubMed] [Google Scholar]
- 66.Terlikowska K. M., Witkowska A. M., Zujko M. E., Dobrzycka B., Terlikowski S. J. Potential application of curcumin and its analogues in the treatment strategy of patients with primary epithelial ovarian cancer. International Journal of Molecular Sciences. 2014;15(12):21703–21722. doi: 10.3390/ijms151221703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ak T., Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chemico-Biological Interactions. 2008;174(1):27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 68.Barzegar A., Moosavi-Movahedi A. A. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PloS ONE. 2011;6(10, article e26012) doi: 10.1371/journal.pone.0026012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim J. E., Kim A. R., Chung H. Y., Han S. Y., Kim B. S., Choi J. S. In vitro peroxynitrite scavenging activity of diarylheptanoids from Curcuma longa. Phytotherapy Research. 2003;17(5):481–484. doi: 10.1002/ptr.1179. [DOI] [PubMed] [Google Scholar]
- 70.McNally S. J., Harrison E. M., Ross J. A., Garden O. J., Wigmore S. J. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. International Journal of Molecular Medicine. 2007;19(1):165–172. [PubMed] [Google Scholar]
- 71.Panchal H. D., Vranizan K., Lee C. Y., Ho J., Ngai J., Timiras P. S. Early anti-oxidative and anti-proliferative curcumin effects on neuroglioma cells suggest therapeutic targets. Neurochemical Research. 2008;33(9):1701–1710. doi: 10.1007/s11064-008-9608-x. [DOI] [PubMed] [Google Scholar]
- 72.Balogun E., Hoque M., Gong P., et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. The Biochemical Journal. 2003;371(3):887–895. doi: 10.1042/bj20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karmakar S., Banik N. L., Patel S. J., Ray S. K. Curcumin activated both receptor-mediated and mitochondria-mediated proteolytic pathways for apoptosis in human glioblastoma T98G cells. Neuroscience Letters. 2006;407(1):53–58. doi: 10.1016/j.neulet.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 74.Gogada R., Amadori M., Zhang H., et al. Curcumin induces Apaf-1-dependent, p21-mediated caspase activation and apoptosis. Cell Cycle. 2011;10(23):4128–4137. doi: 10.4161/cc.10.23.18292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jana N. R., Dikshit P., Goswami A., Nukina N. Inhibition of proteasomal function by curcumin induces apoptosis through mitochondrial pathway. The Journal of Biological Chemistry. 2004;279(12):11680–11685. doi: 10.1074/jbc.M310369200. [DOI] [PubMed] [Google Scholar]
- 76.Morin D., Barthelemy S., Zini R., Labidalle S., Tillement J. P. Curcumin induces the mitochondrial permeability transition pore mediated by membrane protein thiol oxidation. FEBS Letters. 2001;495(1-2):131–136. doi: 10.1016/S0014-5793(01)02376-6. [DOI] [PubMed] [Google Scholar]
- 77.Thayyullathil F., Chathoth S., Hago A., Patel M., Galadari S. Rapid reactive oxygen species (ROS) generation induced by curcumin leads to caspase-dependent and -independent apoptosis in L929 cells. Free Radical Biology & Medicine. 2008;45(10):1403–1412. doi: 10.1016/j.freeradbiomed.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 78.Bhaumik S., Anjum R., Rangaraj N., Pardhasaradhi B. V. V., Khar A. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Letters. 1999;456(2):311–314. doi: 10.1016/S0014-5793(99)00969-2. [DOI] [PubMed] [Google Scholar]
- 79.Watson J. L., Hill R., Yaffe P. B., et al. Curcumin causes superoxide anion production and p53-independent apoptosis in human colon cancer cells. Cancer Letters. 2010;297(1):1–8. doi: 10.1016/j.canlet.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 80.Cao J., Jia L., Zhou H. M., Liu Y., Zhong L. F. Mitochondrial and nuclear DNA damage induced by curcumin in human hepatoma G2 cells. Toxicological Sciences. 2006;91(2):476–483. doi: 10.1093/toxsci/kfj153. [DOI] [PubMed] [Google Scholar]
- 81.Cao J., Liu Y., Jia L., et al. Curcumin induces apoptosis through mitochondrial hyperpolarization and mtDNA damage in human hepatoma G2 cells. Free Radical Biology & Medicine. 2007;43(6):968–975. doi: 10.1016/j.freeradbiomed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 82.Jung E. M., Lim J. H., Lee T. J., Park J. W., Choi K. S., Kwon T. K. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5) Carcinogenesis. 2005;26(11):1905–1913. doi: 10.1093/carcin/bgi167. [DOI] [PubMed] [Google Scholar]
- 83.Yang X., Li Z., Wu Q., Chen S., Yi C., Gong C. TRAIL and curcumin codelivery nanoparticles enhance TRAIL-induced apoptosis through upregulation of death receptors. Drug Delivery. 2017;24(1):1526–1536. doi: 10.1080/10717544.2017.1384863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee Y. J., Kim N. Y., Suh Y. A., Lee C. Involvement of ROS in curcumin-induced autophagic cell death. The Korean Journal of Physiology and Pharmacology. 2011;15(1):1–7. doi: 10.4196/kjpp.2011.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gersey Z. C., Rodriguez G. A., Barbarite E., et al. Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer. 2017;17(1):p. 99. doi: 10.1186/s12885-017-3058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marquardt J. U., Gomez-Quiroz L., Arreguin Camacho L. O., et al. Curcumin effectively inhibits oncogenic NF-κB signaling and restrains stemness features in liver cancer. Journal of Hepatology. 2015;63(3):661–669. doi: 10.1016/j.jhep.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choudhuri T., Pal S., Agwarwal M. L., Das T., Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Letters. 2002;512(1-3):334–340. doi: 10.1016/S0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- 88.Shankar S., Srivastava R. K. Bax and Bak genes are essential for maximum apoptotic response by curcumin, a polyphenolic compound and cancer chemopreventive agent derived from turmeric, Curcuma longa. Carcinogenesis. 2007;28(6):1277–1286. doi: 10.1093/carcin/bgm024. [DOI] [PubMed] [Google Scholar]
- 89.Yang J., Ning J., Peng L., He D. Effect of curcumin on Bcl-2 and Bax expression in nude mice prostate cancer. International Journal of Clinical and Experimental Pathology. 2015;8(8):9272–9278. [PMC free article] [PubMed] [Google Scholar]
- 90.Yu J., Zhou X., He X., Dai M., Zhang Q. Curcumin induces apoptosis involving bax/bcl-2 in human hepatoma SMMC-7721 cells. Asian Pacific Journal of Cancer Prevention. 2011;12(8):1925–1929. [PubMed] [Google Scholar]
- 91.Kim S. Y., Jung S. H., Kim H. S. Curcumin is a potent broad spectrum inhibitor of matrix metalloproteinase gene expression in human astroglioma cells. Biochemical and Biophysical Research Communications. 2005;337(2):510–516. doi: 10.1016/j.bbrc.2005.09.079. [DOI] [PubMed] [Google Scholar]
- 92.Kumar D., Kumar M., Saravanan C., Singh S. K. Curcumin: a potential candidate for matrix metalloproteinase inhibitors. Expert Opinion on Therapeutic Targets. 2012;16(10):959–972. doi: 10.1517/14728222.2012.710603. [DOI] [PubMed] [Google Scholar]
- 93.Aza-Gonzalez C., Nunez-Palenius H. G., Ochoa-Alejo N. Molecular biology of capsaicinoid biosynthesis in chili pepper (Capsicum spp.) Plant Cell Reports. 2011;30(5):695–706. doi: 10.1007/s00299-010-0968-8. [DOI] [PubMed] [Google Scholar]
- 94.Bley K., Boorman G., Mohammad B., McKenzie D., Babbar S. A comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicin. Toxicologic Pathology. 2012;40(6):847–873. doi: 10.1177/0192623312444471. [DOI] [PubMed] [Google Scholar]
- 95.Lu H. F., Chen Y. L., Yang J. S., et al. Antitumor activity of capsaicin on human colon cancer cells in vitro and colo 205 tumor xenografts in vivo. Journal of Agricultural and Food Chemistry. 2010;58(24):12999–13005. doi: 10.1021/jf103335w. [DOI] [PubMed] [Google Scholar]
- 96.Clark R., Lee S. H. Anticancer properties of capsaicin against human cancer. Anticancer Research. 2016;36(3):837–843. [PubMed] [Google Scholar]
- 97.Impheng H., Pongcharoen S., Richert L., Pekthong D., Srisawang P. The selective target of capsaicin on FASN expression and de novo fatty acid synthesis mediated through ROS generation triggers apoptosis in HepG2 cells. PLoS ONE. 2014;9(9, article e107842) doi: 10.1371/journal.pone.0107842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang K. M., Pyo J. O., Kim G. Y., et al. Capsaicin induces apoptosis by generating reactive oxygen species and disrupting mitochondrial transmembrane potential in human colon cancer cell lines. Cellular & Molecular Biology Letters. 2009;14(3):497–510. doi: 10.2478/s11658-009-0016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang R., Humphreys I., Sahu R. P., Shi Y., Srivastava S. K. In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis. 2008;13(12):1465–1478. doi: 10.1007/s10495-008-0278-6. [DOI] [PubMed] [Google Scholar]
- 100.Xie L., Xiang G. H., Tang T., et al. Capsaicin and dihydrocapsaicin induce apoptosis in human glioma cells via ROS and Ca2+mediated mitochondrial pathway. Molecular Medicine Reports. 2016;14(5):4198–4208. doi: 10.3892/mmr.2016.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sanchez A. M., Sanchez M. G., Malagarie-Cazenave S., Olea N., Diaz-Laviada I. Induction of apoptosis in prostate tumor PC-3 cells and inhibition of xenograft prostate tumor growth by the vanilloid capsaicin. Apoptosis. 2006;11(1):89–99. doi: 10.1007/s10495-005-3275-z. [DOI] [PubMed] [Google Scholar]
- 102.Macho A., Calzado M. A., Muñoz-Blanco J., et al. Selective induction of apoptosis by capsaicin in transformed cells: the role of reactive oxygen species and calcium. Cell Death and Differentiation. 1999;6(2):155–165. doi: 10.1038/sj.cdd.4400465. [DOI] [PubMed] [Google Scholar]
- 103.Pramanik K. C., Boreddy S. R., Srivastava S. K. Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PLoS ONE. 2011;6(5, article e20151) doi: 10.1371/journal.pone.0020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Amantini C., Mosca M., Nabissi M., et al. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. Journal of Neurochemistry. 2007;102(3):977–990. doi: 10.1111/j.1471-4159.2007.04582.x. [DOI] [PubMed] [Google Scholar]
- 105.Amantini C., Ballarini P., Caprodossi S., et al. Triggering of transient receptor potential vanilloid type 1 (TRPV1) by capsaicin induces Fas/CD95-mediated apoptosis of urothelial cancer cells in an ATM-dependent manner. Carcinogenesis. 2009;30(8):1320–1329. doi: 10.1093/carcin/bgp138. [DOI] [PubMed] [Google Scholar]
- 106.Kim S. R., Kim S. U., Oh U., Jin B. K. Transient receptor potential vanilloid subtype 1 mediates microglial cell death in vivo and in vitro via Ca2+-mediated mitochondrial damage and cytochrome c release. Journal of Immunology. 2006;177(7):4322–4329. doi: 10.4049/jimmunol.177.7.4322. [DOI] [PubMed] [Google Scholar]
- 107.Chen D., Yang Z., Wang Y., Zhu G., Wang X. Capsaicin induces cycle arrest by inhibiting cyclin-dependent-kinase in bladder carcinoma cells. International Journal of Urology. 2012;19(7):662–668. doi: 10.1111/j.1442-2042.2012.02981.x. [DOI] [PubMed] [Google Scholar]
- 108.Lin C. H., Lu W. C., Wang C. W., Chan Y. C., Chen M. K. Capsaicin induces cell cycle arrest and apoptosis in human KB cancer cells. BMC Complementary and Alternative Medicine. 2013;13(1) doi: 10.1186/1472-6882-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Min K. J., Kwon T. K. Anticancer effects and molecular mechanisms of epigallocatechin-3-gallate. Integrative Medicine Research. 2014;3(1):16–24. doi: 10.1016/j.imr.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nakazato T., Ito K., Miyakawa Y., et al. Catechin, a green tea component, rapidly induces apoptosis of myeloid leukemic cells via modulation of reactive oxygen species production in vitro and inhibits tumor growth in vivo. Haematologica. 2005;90(3):317–325. [PubMed] [Google Scholar]
- 111.Noda C., He J., Takano T., et al. Induction of apoptosis by epigallocatechin-3-gallate in human lymphoblastoid B cells. Biochemical and biophysical research communications. 2007;362(4):951–957. doi: 10.1016/j.bbrc.2007.08.079. [DOI] [PubMed] [Google Scholar]
- 112.Li W., Nie S., Yu Q., Xie M. (-)-Epigallocatechin-3-gallate induces apoptosis of human hepatoma cells by mitochondrial pathways related to reactive oxygen species. Journal of agricultural and food chemistry. 2009;57(15):6685–6691. doi: 10.1021/jf901396f. [DOI] [PubMed] [Google Scholar]
- 113.Liu S., Wang X. J., Liu Y., Cui Y. F. PI3K/AKT/mTOR signaling is involved in (-)-epigallocatechin-3-gallate-induced apoptosis of human pancreatic carcinoma cells. The American journal of Chinese medicine. 2013;41(03):629–642. doi: 10.1142/S0192415X13500444. [DOI] [PubMed] [Google Scholar]
- 114.Gu J. J., Qiao K. S., Sun P., Chen P., Li Q. Study of EGCG induced apoptosis in lung cancer cells by inhibiting PI3K/Akt signaling pathway. European review for medical and pharmacological sciences. 2018;22(14):4557–4563. doi: 10.26355/eurrev_201807_15511. [DOI] [PubMed] [Google Scholar]
- 115.Wu P. P., Kuo S. C., Huang W. W., et al. Epigallocatechin gallate induced apoptosis in human adrenal cancer NCI-H295 cells through caspase-dependent and caspase-independent pathway. Anticancer research. 2009;29(4):1435–1442. [PubMed] [Google Scholar]
- 116.Shankar S., Suthakar G., Srivastava R. K. Epigallocatechin-3-gallate inhibits cell cycle and induces apoptosis in pancreatic cancer. Frontiers in Bioscience. 2007;12(12):5039–5051. doi: 10.2741/2446. [DOI] [PubMed] [Google Scholar]
- 117.Qin J., Xie L. P., Zheng X. Y., et al. A component of green tea, (-)-epigallocatechin-3-gallate, promotes apoptosis in T24 human bladder cancer cells via modulation of the PI3K/Akt pathway and Bcl-2 family proteins. Biochemical and biophysical research communications. 2007;354(4):852–857. doi: 10.1016/j.bbrc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 118.Chung S. S., Vadgama J. V. Curcumin and Epigallocatechin Gallate Inhibit the Cancer Stem Cell Phenotype via Down-regulation of STAT3–NFκB Signaling. Anticancer Research. 2015;35(1):39–46. [PMC free article] [PubMed] [Google Scholar]
- 119.Chou Y. C., Chang M. Y., Wang M. J., et al. PEITC induces apoptosis of human brain glioblastoma GBM8401 cells through the extrinsic- and intrinsic -signaling pathways. Neurochemistry international. 2015;81:32–40. doi: 10.1016/j.neuint.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 120.Gupta P., Adkins C., Lockman P., Srivastava S. K. Metastasis of breast tumor cells to brain is suppressed by phenethyl isothiocyanate in a novel in vivo metastasis model. PLoS ONE. 2013;8(6, article e67278) doi: 10.1371/journal.pone.0067278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kalkunte S., Swamy N., Dizon D. S., Brard L. Benzyl isothiocyanate (BITC) induces apoptosis in ovarian cancer cells in vitro. Journal of experimental therapeutics & oncology. 2006;5(4):287–300. [PubMed] [Google Scholar]
- 122.Zhu Y., Zhuang J. X., Wang Q., Zhang H. Y., Yang P. Inhibitory effect of benzyl isothiocyanate on proliferation in vitro of human glioma cells. Asian Pacific journal of cancer prevention. 2013;14(4):2607–2610. doi: 10.7314/APJCP.2013.14.4.2607. [DOI] [PubMed] [Google Scholar]
- 123.Higdon J. V., Delage B., Williams D. E., Dashwood R. H. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacological Research. 2007;55(3):224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tang L., Zirpoli G. R., Guru K., et al. Intake of cruciferous vegetables modifies bladder cancer survival. Cancer Epidemiology Biomarkers & Prevention. 2010;19(7):1806–1811. doi: 10.1158/1055-9965.EPI-10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xiao D., Powolny A. A., Moura M. B., et al. Phenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cells. The Journal of biological chemistry. 2010;285(34):26558–26569. doi: 10.1074/jbc.M109.063255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Trachootham D., Zhou Y., Zhang H., et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10(3):241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 127.Xiao D., Vogel V., Singh S. V. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Molecular Cancer Therapeutics. 2006;5(11):2931–2945. doi: 10.1158/1535-7163.MCT-06-0396. [DOI] [PubMed] [Google Scholar]
- 128.Wang J., Luo B., Li X., et al. Inhibition of cancer growth in vitro and in vivo by a novel ROS-modulating agent with ability to eliminate stem-like cancer cells. Cell death & disease. 2017;8(6, article e2887) doi: 10.1038/cddis.2017.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hong Y.-H., Uddin H., Jo U., et al. ROS accumulation by PEITC selectively kills ovarian cancer cells via UPR-mediated apoptosis. Frontiers in oncology. 2015;5:p. 167. doi: 10.3389/fonc.2015.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y., Wei S., Wang J., Fang Q., Chai Q. Phenethyl isothiocyanate inhibits growth of human chronic myeloid leukemia K562 cells via reactive oxygen species generation and caspases. Molecular medicine reports. 2014;10(1):543–549. doi: 10.3892/mmr.2014.2167. [DOI] [PubMed] [Google Scholar]
- 131.Kasiappan R., Jutooru I., Karki K., Hedrick E., Safe S. Benzyl isothiocyanate (BITC) induces reactive oxygen species-dependent repression of STAT3 protein by down-regulation of specificity proteins in pancreatic cancer. The Journal of biological chemistry. 2016;291(53):27122–27133. doi: 10.1074/jbc.M116.746339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin J. F., Tsai T. F., Yang S. C., et al. Benzyl isothiocyanate induces reactive oxygen species-initiated autophagy and apoptosis in human prostate cancer cells. Oncotarget. 2017;8(12):20220–20234. doi: 10.18632/oncotarget.15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ranjan A., Fofaria N. M., Kim S. H., Srivastava S. K. Modulation of signal transduction pathways by natural compounds in cancer. Chinese journal of natural medicines. 2015;13(10):730–742. doi: 10.1016/S1875-5364(15)30073-X. [DOI] [PubMed] [Google Scholar]
- 134.Sahu R. P., Zhang R., Batra S., Shi Y., Srivastava S. K. Benzyl isothiocyanate-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of MAPK in human pancreatic cancer cells. Carcinogenesis. 2009;30(10):1744–1753. doi: 10.1093/carcin/bgp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yeh Y. T., Yeh H., Su S. H., et al. Phenethyl isothiocyanate induces DNA damage-associated G2/M arrest and subsequent apoptosis in oral cancer cells with varying p53 mutations. Free radical biology & medicine. 2014;74:1–13. doi: 10.1016/j.freeradbiomed.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 136.Zhang R., Loganathan S., Humphreys I., Srivastava S. K. Benzyl isothiocyanate-induced DNA damage causes G2/M cell cycle arrest and apoptosis in human pancreatic cancer cells. The Journal of Nutrition. 2006;136(11):2728–2734. doi: 10.1093/jn/136.11.2728. [DOI] [PubMed] [Google Scholar]
- 137.Rather R. A., Bhagat M. Cancer chemoprevention and piperine: molecular mechanisms and therapeutic opportunities. Frontiers in cell and developmental biology. 2018;6:p. 10. doi: 10.3389/fcell.2018.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gunasekaran V., Elangovan K., Niranjali Devaraj S. Targeting hepatocellular carcinoma with piperine by radical-mediated mitochondrial pathway of apoptosis: an in vitro and in vivo study. Food and Chemical Toxicology. 2017;105:106–118. doi: 10.1016/j.fct.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 139.Siddiqui S., Ahamad M. S., Jafri A., Afzal M., Arshad M. Piperine triggers apoptosis of human oral squamous carcinoma through cell cycle arrest and mitochondrial oxidative stress. Nutrition and Cancer. 2017;69(5):791–799. doi: 10.1080/01635581.2017.1310260. [DOI] [PubMed] [Google Scholar]
- 140.Takashina M., Inoue S., Tomihara K., et al. Different effect of resveratrol to induction of apoptosis depending on the type of human cancer cells. International Journal of Oncology. 2017;50(3):787–797. doi: 10.3892/ijo.2017.3859. [DOI] [PubMed] [Google Scholar]
- 141.Hsieh T. C., Wu J. M. Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines. Experimental Cell Research. 1999;249(1):109–115. doi: 10.1006/excr.1999.4471. [DOI] [PubMed] [Google Scholar]
- 142.Chen Y., Tseng S. H., Lai H. S., Chen W. J. Resveratrol-induced cellular apoptosis and cell cycle arrest in neuroblastoma cells and antitumor effects on neuroblastoma in mice. Surgery. 2004;136(1):57–66. doi: 10.1016/j.surg.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 143.Shankar S., Chen Q., Siddiqui I., Sarva K., Srivastava R. K. Sensitization of TRAIL-resistant LNCaP cells by resveratrol (3, 4′, 5 tri-hydroxystilbene): molecular mechanisms and therapeutic potential. Journal of Molecular Signaling. 2007;2:p. 7. doi: 10.1186/1750-2187-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Varoni E. M., Lo Faro A. F., Sharifi-Rad J., Iriti M. Anticancer molecular mechanisms of resveratrol. Frontiers in Nutrition. 2016;3 doi: 10.3389/fnut.2016.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Harikumar K. B., Aggarwal B. B. Resveratrol: A multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7(8):1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 146.Whitlock N. C., Baek S. J. The anticancer effects of resveratrol: modulation of transcription factors. Nutrition and Cancer. 2012;64(4):493–502. doi: 10.1080/01635581.2012.667862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gledhill J. R., Montgomery M. G., Leslie A. G. W., Walker J. E. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proceedings of the National Academy of Sciences. 2007;104(34):13632–13637. doi: 10.1073/pnas.0706290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zheng J., Ramirez V. D. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. British Journal of Pharmacology. 2000;130(5):1115–1123. doi: 10.1038/sj.bjp.0703397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Singh S. K., Banerjee S., Acosta E. P., Lillard J. W., Singh R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/ p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget. 2017;8(10):17216–17228. doi: 10.18632/oncotarget.15303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Athar M., Back J. H., Kopelovich L., Bickers D. R., Kim A. L. Multiple molecular targets of resveratrol: anti-carcinogenic mechanisms. Archives of Biochemistry and Biophysics. 2009;486(2):95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rosa L. D. S., Jordão N. A., Soares N. D. C. P., DeMesquita J. F., Monteiro M., Teodoro A. J. Pharmacokinetic, Antiproliferative and Apoptotic Effects of Phenolic Acids in Human Colon Adenocarcinoma Cells Using In Vitro and In Silico Approaches. Molecules. 2018;23(10):p. 2569. doi: 10.3390/molecules23102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jaganathan S. K., Supriyanto E., Mandal M. Events associated with apoptotic effect of p-Coumaric acid in HCT-15 colon cancer cells. World Journal of Gastroenterology. 2013;19(43):7726–7734. doi: 10.3748/wjg.v19.i43.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sharma S. H., Rajamanickam V., Nagarajan S. Antiproliferative effect of p-Coumaric acid targets UPR activation by downregulating Grp78 in colon cancer. Chemico-Biological Interactions. 2018;291:16–28. doi: 10.1016/j.cbi.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 154.Ong J. Y. H., Yong P. V. C., Lim Y. M., Ho A. S. H. 2-Methoxy-1,4-naphthoquinone (MNQ) induces apoptosis of A549 lung adenocarcinoma cells via oxidation-triggered JNK and p38 MAPK signaling pathways. Life Sciences. 2015;135:158–164. doi: 10.1016/j.lfs.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 155.IgnacioAguiló J., Iturralde M., Monleón I., et al. Cytotoxicity of quinone drugs on highly proliferative human leukemia T cells: reactive oxygen species generation and inactive shortened SOD1 isoform implications. Chemico-Biological Interactions. 2012;198(1-3):18–28. doi: 10.1016/j.cbi.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 156.Lee E. B., Cheon M. G., Cui J., Lee Y. J., Seo E. K., Jang H. H. The quinone-based derivative, HMNQ induces apoptotic and autophagic cell death by modulating reactive oxygen species in cancer cells. Oncotarget. 2017;8(59):99637–99648. doi: 10.18632/oncotarget.21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee E. R., Kang Y. J., Kim H. J., et al. Regulation of apoptosis by modified naringenin derivatives in human colorectal carcinoma RKO cells. Journal of Cellular Biochemistry. 2008;104(1):259–273. doi: 10.1002/jcb.21622. [DOI] [PubMed] [Google Scholar]
- 158.Totta P., Acconcia F., Leone S., Cardillo I., Marino M. Mechanisms of Naringenin-induced Apoptotic Cascade in Cancer Cells: Involvement of Estrogen Receptor α and β Signalling. IUBMB Life. 2004;56(8):491–499. doi: 10.1080/15216540400010792. [DOI] [PubMed] [Google Scholar]
- 159.You B. R., Kim S. Z., Kim S. H., Park W. H. Gallic acid-induced lung cancer cell death is accompanied by ROS increase and glutathione depletion. Molecular and Cellular Biochemistry. 2011;357(1-2):295–303. doi: 10.1007/s11010-011-0900-8. [DOI] [PubMed] [Google Scholar]
- 160.Russell JR L. H., Mazzio E., Badisa R. B., et al. Autoxidation of gallic acid induces ROS-dependent death in human prostate cancer LNCaP cells. Anticancer Research. 2012;32(5):1595–1602. [PMC free article] [PubMed] [Google Scholar]
- 161.Chuang C. Y., Liu H. C., Wu L. C., Chen C. Y., Chang J. T., Hsu S. L. Gallic acid induces apoptosis of lung fibroblasts via a reactive oxygen species-dependent ataxia telangiectasia mutated-p53 activation pathway. Journal of Agricultural and Food Chemistry. 2010;58(5):2943–2951. doi: 10.1021/jf9043265. [DOI] [PubMed] [Google Scholar]
- 162.Chen C. Y., Chen K. C., Yang T. Y., Liu H. C., Hsu S. L. Gallic acid induces a reactive oxygen species-provoked c-Jun NH2-terminal kinase-dependent apoptosis in lung fibroblasts. Evidence-Based Complementary and Alternative Medicine. 2013;2013:12. doi: 10.1155/2013/613950.613950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen X., Yin O. Q. P., Zuo Z., Chow M. S. S. Pharmacokinetics and modeling of quercetin and metabolites. Pharmaceutical Research. 2005;22(6):892–901. doi: 10.1007/s11095-005-4584-1. [DOI] [PubMed] [Google Scholar]
- 164.Vareed S. K., Kakarala M., Ruffin M. T., et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiology Biomarkers & Prevention. 2008;17(6):1411–1417. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Walle T. Bioavailability of resveratrol. Annals of the New York Academy of Sciences. 2011;1215(1):9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- 166.Burgos-Moron E., Calderon-Montano J. M., Salvador J., Robles A., Lopez-Lazaro M. The dark side of curcumin. International Journal of Cancer. 2010;126(7):1771–1775. doi: 10.1002/ijc.24967. [DOI] [PubMed] [Google Scholar]
- 167.Shoba G., Joy D., Joseph T., Majeed M., Rajendran R., Srinivas P. S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Medica. 1998;64(04):353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 168.Johnson J. J., Nihal M., Siddiqui I. A., et al. Enhancing the bioavailability of resveratrol by combining it with piperine. Molecular Nutrition & Food Research. 2011;55(8):1169–1176. doi: 10.1002/mnfr.201100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vyas A., Dandawate P., Padhye S., Ahmad A., Sarkar F. Perspectives on new synthetic curcumin analogs and their potential anticancer properties. Current Pharmaceutical Design. 2013;19(11):2047–2069. [PMC free article] [PubMed] [Google Scholar]
- 170.Cai X., Fang Z., Dou J., Yu A., Zhai G. Bioavailability of quercetin: problems and promises. Current Medicinal Chemistry. 2013;20(20):2572–2582. doi: 10.2174/09298673113209990120. [DOI] [PubMed] [Google Scholar]
- 171.Urbina-Cano P., Bobadilla-Morales L., Ramírez-Herrera M. A., et al. DNA damage in mouse lymphocytes exposed to curcumin and copper. Journal of Applied Genetics. 2006;47(4):377–382. doi: 10.1007/BF03194648. [DOI] [PubMed] [Google Scholar]
- 172.Blasiak J., Trzeciak A., Kowalik J. Curcumin damages DNA in human gastric mucosa cells and lymphocytes. Journal of Environmental Pathology, Toxicology and Oncology. 1999;18:271–276. [PubMed] [Google Scholar]
- 173.Dance-Barnes S. T., Kock N. D., Moore J. E., et al. Lung tumor promotion by curcumin. Carcinogenesis. 2009;30(6):1016–1023. doi: 10.1093/carcin/bgp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bode A. M., Dong Z. The two faces of capsaicin. Cancer Research. 2011;71(8):2809–2814. doi: 10.1158/0008-5472.CAN-10-3756. [DOI] [PubMed] [Google Scholar]
- 175.Hu J., Chen L., Lei F., et al. Investigation of quercetin stability in cell culture medium: role in in vitro experiment. African Journal of Pharmacy and Pharmacology. 2012;6:1069–1076. [Google Scholar]
- 176.Savale S. K. Curcumin as a model drug: conformation, solubility estimation, morphological, in vitro and in vivo biodistribution study. Journal of PharmaSciTech. 2017;7:31–35. [Google Scholar]
- 177.Kocyigit A., Guler E. M., Dikilitas M. Reactive Oxygen Species (ROS) in Living Cells. IntechOpen; 2017. Role of antioxidant phytochemicals in prevention, formation and treatment of cancer. [Google Scholar]
- 178.Hashemzaei M., Far A. D., Yari A., et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncology Reports. 2017;38(2):819–828. doi: 10.3892/or.2017.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pratheeshkumar P., Budhraja A., Son Y. O., et al. Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR- 2 regulated AKT/mTOR/P70S6K signaling pathways. PLoS ONE. 2012;7(10, article e47516) doi: 10.1371/journal.pone.0047516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhou J., Fang L., Liao J., et al. Investigation of the anti-cancer effect of quercetin on HepG2 cells in vivo. PLoS ONE. 2017;12(3, article e0172838) doi: 10.1371/journal.pone.0172838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maso V., Calgarotto A. K., Franchi G. C., et al. Multitarget effects of quercetin in leukemia. Cancer Prevention Research. 2014;7(12):1240–1250. doi: 10.1158/1940-6207.CAPR-13-0383. [DOI] [PubMed] [Google Scholar]
- 182.Lu W. D., Qin Y., Yang C., Li L., Fu Z. X. Effect of curcumin on human colon cancer multidrug resistance in vitro and in vivo. Clinics. 2013;68(5):694–701. doi: 10.6061/clinics/2013(05)18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lai H. W., Chien S. Y., Kuo S. J., et al. The Potential Utility of Curcumin in the Treatment of HER-2-Overexpressed Breast Cancer: AnIn VitroandIn VivoComparison Study with Herceptin. Evidence-Based Complementary and Alternative Medicine. 2012;2012:12. doi: 10.1155/2012/486568.486568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mori A., Lehmann S., O'Kelly J., et al. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Research. 2006;66(6):3222–3229. doi: 10.1158/0008-5472.CAN-05-0087. [DOI] [PubMed] [Google Scholar]
- 185.Thoennissen N. H., O'Kelly J., Lu D., et al. Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and -negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene. 2010;29(2):285–296. doi: 10.1038/onc.2009.335. [DOI] [PubMed] [Google Scholar]
- 186.Shin Y. S., Kang S. U., Park J. K., et al. Anti-cancer effect of (-)-epigallocatechin-3-gallate (EGCG) in head and neck cancer through repression of transactivation and enhanced degradation of β-catenin. Phytomedicine. 2016;23(12):1344–1355. doi: 10.1016/j.phymed.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 187.Luo K. W., Lung W. Y., C. X., Luo X. L., Huang W. R. EGCG inhibited bladder cancer T24 and 5637 cell proliferation and migration via PI3K/AKT pathway. Oncotarget. 2018;9(15):12261–12272. doi: 10.18632/oncotarget.24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sakamoto Y., Terashita N., Muraguchi T., Fukusato T., Kubota S. Effects of epigallocatechin-3-gallate (EGCG) on A549 lung cancer tumor growth and angiogenesis. Bioscience, Biotechnology, and Biochemistry. 2013;77(9):1799–1803. doi: 10.1271/bbb.120882. [DOI] [PubMed] [Google Scholar]
- 189.Hu Q., Chang X., Yan R., et al. (-)-Epigallocatechin-3-gallate induces cancer cell apoptosis via acetylation of amyloid precursor protein. Medical Oncology. 2015;32(1):p. 390. doi: 10.1007/s12032-014-0390-0. [DOI] [PubMed] [Google Scholar]
- 190.Chou Y. C., Chang M. Y., Lee H. T., et al. Phenethyl isothiocyanate inhibits in vivo growth of xenograft tumors of human glioblastoma cells. Molecules. 2018;23(9):p. 2305. doi: 10.3390/molecules23092305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xiao D., Lew K. L., Zeng Y., et al. Phenethyl isothiocyanate-induced apoptosis in PC-3 human prostate cancer cells is mediated by reactive oxygen species-dependent disruption of the mitochondrial membrane potential. Carcinogenesis. 2006;27(11):2223–2234. doi: 10.1093/carcin/bgl087. [DOI] [PubMed] [Google Scholar]
- 192.Gupta P., Srivastava S. K. Antitumor activity of phenethyl isothiocyanate in HER2-positive breast cancer models. BMC Medicine. 2012;10(1) doi: 10.1186/1741-7015-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Loganathan S., Kandala P. K., Gupta P., Srivastava S. K. Inhibition of EGFR-AKT axis results in the suppression of ovarian tumors in vitro and in preclinical mouse model. PloS ONE. 2012;7(8, article e43577) doi: 10.1371/journal.pone.0043577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lai L. H., Fu Q. H., Liu Y., et al. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacologica Sinica. 2012;33(4):523–530. doi: 10.1038/aps.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Samykutty A., Shetty A. V., Dakshinamoorthy G., et al. Piperine, a bioactive component of pepper spice exerts therapeutic effects on androgen dependent and androgen independent prostate cancer cells. PloS ONE. 2013;8(6, article e65889) doi: 10.1371/journal.pone.0065889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sun L., Li H., Chen J., et al. A SUMOylation-dependent pathway regulates SIRT1 transcription and lung cancer metastasis. Journal of the National Cancer Institute. 2013;105(12):887–898. doi: 10.1093/jnci/djt118. [DOI] [PubMed] [Google Scholar]
- 197.Bai Y., Mao Q. Q., Qin J., et al. Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro and inhibits tumor growth in vivo. Cancer Science. 2010;101(2):488–493. doi: 10.1111/j.1349-7006.2009.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Garvin S., Ollinger K., Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Letters. 2006;231(1):113–122. doi: 10.1016/j.canlet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 199.Roy S. K., Chen Q., Fu J., Shankar S., Srivastava R. K. Resveratrol inhibits growth of orthotopic pancreatic tumors through activation of FOXO transcription factors. PloS ONE. 2011;6(9, article e25166) doi: 10.1371/journal.pone.0025166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dias S. J., Li K., Rimando A. M., et al. Trimethoxy-resveratrol and piceatannol administered orally suppress and inhibit tumor formation and growth in prostate cancer xenografts. The Prostate. 2013;73(11):1135–1146. doi: 10.1002/pros.22657. [DOI] [PubMed] [Google Scholar]