Short abstract
Background
Chronic pain has been shown to depend on nociceptive sensitization in the spinal cord, and while multiple mechanisms involved in the initiation of plastic changes have been established, the molecular targets which maintain spinal nociceptive sensitization are still largely unknown. Building upon the established neurobiology underlying the maintenance of long-term potentiation in the hippocampus, this present study investigated the contributions of spinal atypical protein kinase C (PKC) isoforms PKCι/λ and PKMζ and their downstream targets (p62/GluA1 and NSF/GluA2 interactions, respectively) to the maintenance of spinal nociceptive sensitization in male and female rats.
Results
Pharmacological inhibition of atypical PKCs by ZIP reversed established allodynia produced by repeated intramuscular acidic saline injections in male animals only, replicating previously demonstrated sex differences. Inhibition of both PKCι/λ and downstream substrates p62/GluA1 resulted in male-specific reversals of intramuscular acidic saline-induced allodynia, while female animals continued to display allodynia. Inhibition of NSF/GluA2, the downstream target to PKMζ, reversed allodynia induced by intramuscular acidic saline in both sexes. Neither PKCι/λ, p62/GluA1 or NSF/GluA2 inhibition had any effect on formalin response for either sex.
Conclusion
This study provides novel behavioural evidence for the male-specific role of PKCι/λ and downstream target p62/GluA1, highlighting the potential influence of ongoing afferent input. The sexually divergent pathways underlying persistent pain are shown here to converge at the interaction between NSF and the GluA2 subunit of the AMPA receptor. Although this interaction is thought to be downstream of PKMζ in males, these findings and previous work suggest that females may rely on a factor independent of atypical PKCs for the maintenance of spinal nociceptive sensitization.
Keywords: Protein kinase M-zeta, protein kinase C iota/lambda, sex differences, central sensitization, allodynia, analgesia
Introduction
The maintenance of chronic pain in spinal cord dorsal horn (SCDH) and memory within the hippocampus share similar underlying molecular mechanisms, including distinct early and late phases underlying the initiation and maintenance of the plastic changes.1–3 Although multiple signalling molecules (PKC, CAMKII, and ERK1,2) involved in the early initiation of spinal nociceptive sensitivity have been established,4–6 the molecular targets that maintain this hypersensitivity remain largely unclear. How nociceptive sensitivity is maintained is thought to contribute to the transition from acute to chronic, persistent pain.2 The established neurobiology of long-term potentiation (LTP) maintenance in the hippocampus may be used as a guide to identify targets involved with the maintenance of plasticity in the SCDH; a critical area of study for understanding the persistence of a centralized pain state and pain chronicity.3,7
Protein kinase M zeta (PKMζ), an autonomously active atypical PKC isoform, has been implicated in the maintenance of hippocampal LTP and memory,8–11 as well as persistent pain states.3,12–16 PKMζ is increased in the SCDH for a prolonged period following intraplantar (i.pl.) injection of formalin or capsaicin, as well as direct stimulation of SCDH nociceptive neurons by spinal injection of the glutamate agonist dihydroxyphenylglycine.12,16 Behavioural and electrophysiological findings supporting the maintenance role of PKMζ have been largely generated using the cell permeable zeta pseudosubstrate inhibitory peptide (ZIP), a decoy peptide that mimics PKMζ’s missing regulatory region that would normally reduce the autonomous activity of the kinase.3,8–16
Notably, the effects of spinal PKMζ inhibition using ZIP appear to be demonstrated only in pain tests which depend primarily on central sensitization. There has been a demonstrated lack of effect of ZIP in pain tests known to be dependent on nociceptor sensitization,12 or those with high ongoing peripheral input, for which reducing central sensitization may not be enough to reduce pain behaviours.3,16–18 As such, hyperalgesic priming, in which an ordinarily sub-threshold challenging stimulus is delivered following the acute sensitization from an initial priming stimulus,19,20 has often been used to isolate the centrally-mediated effects of ZIP.3,13–15
The specificity of ZIP, and consequently, the role of PKMζ, has been recently questioned following genetic ablation studies in the hippocampus.22,23 Constitutive PKMζ (PRKCZ) knockout mice reported no deficits in late-LTP or hippocampal-dependent learning and memory, and administration of ZIP to the PRKCZ knockout mice still reversed late-LTP, learning and memory; suggesting non-specific effects of the peptide inhibitor.22,23 ZIP is a myristoylated autoinhibitory pseudosubstrate amino acid sequence found in the regulatory domains of all atypical PKC isoforms: PKCζ, PKMζ and PKCι/λ. As such, ZIP has been shown to inhibit additional isoforms in in vitro assays,21 as well as in vivo,23,24 and it was proposed that these may be the target of ZIP, responsible for the maintenance of plasticity.23 However, conditional knockout of PRKCZ identified a compensatory maintenance mechanism for LTP by PKCι/λ; proposing that the permanent deletion of the PRKCZ gene through constitutive knockout resulted in a functional redundancy in atypical PKC signalling pathways.24 These authors showed that a selective PKCι/λ-antagonist (ICAP) disrupts late-LTP and spatial memory in PKMζ-null mice, but not in wild-type mice,24 proposing that PKCι/λ compensates for the action of PKMζ, only if it has been deleted.24 Moreover, the distinct atypical PKC pathways mediating AMPA receptor (AMPAR) trafficking in the early and late phases of synaptic potentiation have also been shown to undergo a mechanistic switch following conditional PKCι/λ knockout studies, switching between the established early PKCι/λ-p62/GluA1-driven AMPAR exocytosis and late PKMζ-NSF/GluA2-driven reduction in AMPAR endocytosis,25,26 further demonstrating atypical PKC compensation to maintain memory function.
In contrast to the hippocampal findings, constitutive knockout of PRKCZ was shown to have consistent effects with spinal administration of ZIP in models of neuropathic, inflammatory, and referred muscle and visceral pain.3 However, knowledge of the mechanisms maintaining spinal nociceptive sensitization is far behind that of hippocampal LTP. While it has been established that the atypical isoform PKCζ does not maintain persistent pain states,12 there has been little investigation into the contribution of PKCι/λ or the potential downstream targets of either PKCι/λ or PKMζ (p62/GluA1 and NSF/GluA2, respectively). Moreover, nearly all research on PKMζ and pain has solely used male animals, with the exception of one study.3 Nasir et al.3 demonstrated that both spinal ZIP administration and constitutive PRKCZ ablation reversed allodynia produced by repeated injections of intracolonic capsaicin or intramuscular (i.m.) acidic saline in male rodents, but neither showed a reduction in the persistent allodynia developed in females following the same treatment.3 This sexual divergence underlying the same painful endpoint suggests that females likely rely on mechanisms other than PKMζ for the maintenance of spinal nociceptive sensitization. Given the demonstrated ability of PKCι/λ to maintain late-LTP in PKMζ-null mice,24 it is possible that PKCι/λ, rather than PKMζ, may actively maintain the spinal nociceptive plasticity underlying persistent pain in females.2,7
Given the above outlined issues with the specificity of the aPKC inhibitor ZIP, the lack of research on downstream targets of aPKCs and sex differences in the contribution of aPKCs to the maintenance of spinal nociceptive sensitization, we performed the following three experiments which assessed nociceptive behaviours in rat models of persistent pain: (1) the non-specific pharmacological manipulation of atypical PKCs, (2) the specific pharmacological manipulation of PKCι/λ and downstream target p62/GluA1, and (3) the specific pharmacological manipulation of downstream targets of PKMζ, NSF/GluA2.
Materials and methods
Animals
All procedures performed on rats were approved by the McGill University Animal Care Committee and followed the ethical guidelines of the Canadian Council on Animal Care and the International Association for the Study of Pain.
Female and male Long-Evans hooded rats (eight weeks of age) were supplied by Charles River Laboratories (Saint-Constant, QC), housed locally, and kept on a 12-h dark-light cycle with food and water available ad libitum. Prior to the beginning of experiments, naïve rats were acclimatized to the animal care facility for a period of two to five days and then habituated to the testing apparatus for 30-min per day for three consecutive days.
Drug administration
All drugs were administered in a volume of 20 µL into the intrathecal (i.t.) space by lumbar puncture. Rats were anesthetized with isoflurane (4% induction, 2% maintenance), and a 26-gauge needle was inserted between the L5 and L6 vertebrae into the cauda equina; correct placement was verified by a positive reflexive tail flick at initial needle placement and following drug injection. The following drugs were used: ZIP (Myr-SIYRRGARRWRKL-OH, Tocris), scr-ZIP (Myr-RLYRKRIWRSAGR-OH, Tocris Bioscience, Oakville, ON) both dissolved in sterile water at a dose of 10 nmol,12 ICAP, [4-(5-amino-4-carba-moylimidazol-1-yl)-2,3-dihydroxycyclopentyl] methyl dihydrogen (graciously received from Dr. Robert Farese) dissolved in sterile water at a dose of 20 nmol, Tat-GluA1(L2-3) (YGRKKKRRQRRR-DISPRSLSGR), scr-Tat-GluA1(L2-3) (YGRKKKRRQRRR-IFNSDGAFMF) dissolved in sterile water at a dose of 10 µg23 (Tat-GluA1(L2-3) and scr-Tat-GluA1(L2-3) were synthesized by CanPeptide Inc, Pointe-Claire, QC), myr-pep2m (Myr- KRMKVAKNAQ, Tocris) and myr-pep4c (Myr- KRMKVAKSAQ, Tocris), dissolved in sterile water at a dose of 10 µg.13 I.t. doses were selected from previously reported effective doses or obtained from preliminary studies to assess potential toxicity and/or behavioural impairments.
Von Frey testing
Animals were tested in acrylic test chambers (12 cm W 16 cm L 15 cm H) placed on a 6 6 mm grid wire mesh flooring. Mechanical allodynia was assessed by measuring the 50% mechanical paw withdrawal threshold (PWT), using nylon von Frey filaments (Semmes Weinstein Monofilament Kit, Stoelting, Woodale, IL) applied to the rat hind paw for a maximum of 10s. Beginning with a stimulus intensity of 2 g, the force of the filament used was either increased following a lack of response or decreased after a positive paw withdrawal response. The sequence ended once five responses were recorded following the first positive response, or after three changes in the direction of the pattern of responses. Minimum and maximum intensities were 0.25 g and 15 g, respectively. Based on the response pattern, the PWT was calculated using the following formula: 50% g threshold = (10[xf + kδ])/10,000, where xf = strength (in log units) of von Frey filament applied last; k = value for the pattern of positive/negative responses; δ = mean difference (in log units) between stimuli.27
I.m. acidic saline
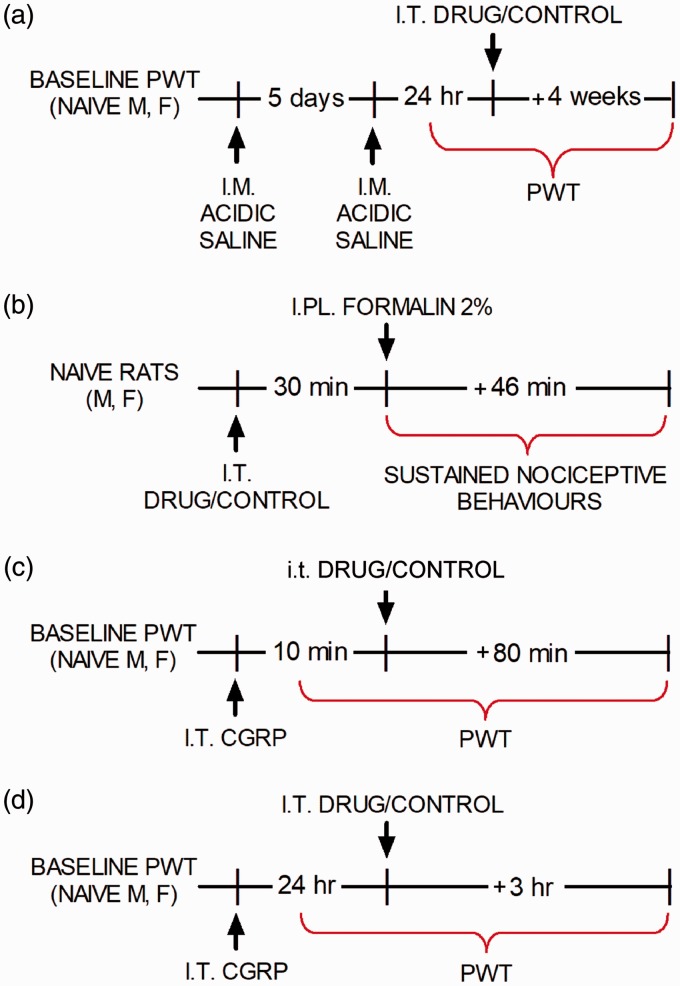
Two i.m. injections of 100 µL of acidic saline (pH adjusted to 4.0) spaced five days apart were administered to the gastrocnemius muscle of naïve rats under brief isoflurane anesthesia (induction 3.5%, maintenance 2%) to produce persistent hind paw mechanical allodynia lasting at least four to five weeks.28 A chart outlining the experimental method is shown in Figure 1(a).
Figure 1.
Experimental design of studies using: (a) intramuscular acidic saline, (b) formalin test, (c) acute intrathecal CGRP and (d) persistent intrathecal CGRP.
PWT: paw withdrawal threshold; i.t.: intrathecal; i.m.: intramuscular; CGRP: calcitonin gene-related peptide.
Formalin test
Rats were tested in acrylic observation boxes (30 cm W 30 cm L 33 cm H) fitted with a mirror placed at 45° underneath the floor to allow for observation of paw placement. On drug test day, rats were pretreated by i.t. injection with the drug or control and returned to the observation box for 30 min. The animals were then restrained by wrapping in a towel, given a 50 µL i.pl. injection of 2% formalin in 0.9% saline to the right hind paw and immediately returned to the observation box for a 46 min period of observation (see Figure 1(b) for experimental design). The biphasic nociceptive response to formalin was assessed by measuring the time rats engaged in each of four categorized behaviours: normal weight bearing on the treated paw (0), reduced weight bearing on the affected paw (1), lifting the treated paw (2), and licking or repeated shaking the injected paw (3). The time engaged in each behavioural category was measured cumulatively for 1 min of every 3 min during the 46-min observation period. A pain score was then computed for each time point by using a weighted means of the measured time spent in each behavioural category.29
I.t. calcitonin gene-related peptide
Acute mechanical allodynia was induced by i.t. injection of calcitonin gene-related peptide (CGRP; 0.5 µM, in a volume of 20 µL) and assessed by von Frey testing first at 10 min and then every 20 min up to 90 min following the injection (see Figure 1(c)). Long-term CGRP-induced allodynia was produced by i.t. injection of a higher dosage (5 µM, 20 µL volume) and assessed by von Frey testing 24 h after injection (see Figure 1(d)).30
Data analysis
All data are presented graphically (Prism 5.0, Graphpad Software, La Jolla, CA) as mean SEM. Drug and sex effects were analyzed across the time course of measurements by repeated measures analysis of variance (ANOVA) (Statistica version 6, Statsoft, Tulsa, OK). A Greenhouse-Geisser correction was applied to the ANOVA tests after the observation of a significant test of sphericity (Mauchley). All reported probabilities therein include Greenhouse-Geisser corrections when applicable. Multiple post-hoc pairwise comparisons were performed using Tukey’s test to assess differences between treatment and control groups at each testing time point, and Dunnett’s t-tests were performed between pre- and post-drug measurements within each group to characterize the time course of treatment effects. To directly compare the response of females and males to the different drug treatments, the area under the curve (AUC) was calculated for each animal in all drug-treated groups using the trapezoidal method applied to the full time course of the post-drug measurements. A two-way ANOVA for independent groups (Sex Drug treatment) was then performed on the AUC values, followed by post-hoc pairwise comparisons using Tukey’s test. In all analyzes, significance was defined as p < 0.05.
Results
Effects of ZIP on allodynia following i.m. acidic saline
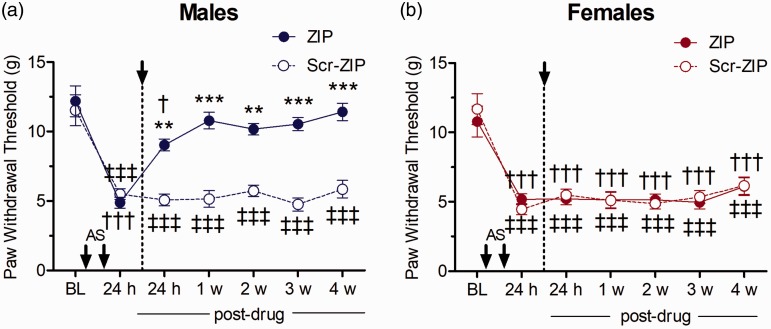
We first examined the role of spinal atypical PKCs (PKMζ, PKCζ, PKCι/λ) in the maintenance of nociceptive sensitization by assessing the post-treatment effects of the non-specific atypical PKC inhibitor ZIP on allodynia produced by repeated i.m. acidic saline injections. Two i.m. injections of acidic saline significantly decreased ipsilateral PWTs from baseline measures in male and female rats, establishing mechanical allodynia in both sexes. There were no differences between males and females in PWTs either before (baseline) or after acidic saline treatment, prior to ZIP administration (Tukey tests, p > 0.05). After treatment, a significant Time Sex Drug interaction (F(6, 120) = 4.0196, p = 0.009) was observed, as the acidic saline-induced mechanical allodynia was reversed by ZIP in males but not in females. The reduction of the mechanical allodynia in males by post-treatment ZIP relative to scrambled peptide treatment was seen at each of the five test time points from 24 h to 4 weeks following drug administration (p = 0.007, p = 0.0002, p= 0.001, p = 0.0002 and p = 0.0002, respectively, Figure 2(a)). In contrast, female rats treated with ZIP displayed significantly reduced PWTs from baseline across the four-week post-treatment period of measurement, and their PWTs were not significantly different from those of control female rats treated with scrambled-ZIP (Tukey test, p > 0.05 at every time point, Figure 2(b)).
Figure 2.
Effect of ZIP (atypical PKC inhibition) on mechanical allodynia after i.m. acidic saline in (a) males and (b) females (n=6/group). Asterisks indicate differences in PWTs between rats treated with ZIP and scrambled control peptide (**p<0.01, ***p<0.001, Tukey test). Daggers indicate differences in PWTs compared to baseline measures for ZIP-treated animals (†††p<0.001, Dunnett test), while double daggers indicate difference from baseline in Scr-ZIP-treated animals (‡‡‡p<0.001, Dunnett test).
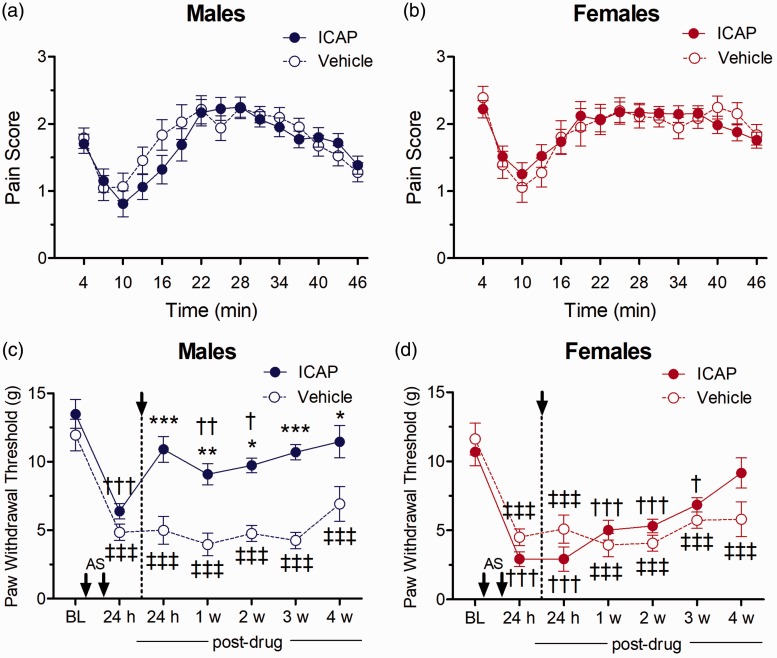
Figure 3.
Effect of ICAP (PKCι/λ inhibition) on nociceptive responses in the formalin test in (a) males (ICAP n=8; VEH n= 7) and (b) females (ICAP n=8; VEH n=6) or on mechanical allodynia after i.m. acidic saline in (c) males and (d) females (n=6/group). Asterisks indicate significant differences in responses between ICAP- and vehicle control-treated rats (*p<0.05, ***p< 0.001, Tukey test). Daggers indicate differences in PWTs compared to baseline measures for ICAP-treated animals (†p<0.05, †††p<0.001, Dunnett test), while double daggers indicate difference from baseline in vehicle-treated animals (‡‡‡p<0.001, Dunnett test).
ICAP: PKCι/λ-antagonist.
Effects of ICAP in the formalin test and following i.m. acidic saline
We then examined the specific contributions of the atypical PKC isoform PKCι/λ to spinal nociceptive processing in the formalin test. PKCι/λ was inhibited by the cell permeable specific PKCι/λ antagonist ICAP24 administered by i.t. injection 25 min prior to formalin. The formalin test produced typical biphasic nociceptive responses over time in both female and male rats. Pre-treatment spinal inhibition of PKCι/λ by ICAP did not elicit significantly different nociceptive responses in the formalin test compared to the behaviours produced by pre-treatment of the vehicle control in either male (Figure 3(a)) or female rats (Figure 3(b)), and thus no sex differences occurred.
We next examined the effects of the ICAP on allodynia produced by i.m. acidic saline. Mechanical allodynia was established in both sexes by the i.m. acidic saline injections, with PWTs significantly reduced from baseline levels prior to ICAP treatment. There were no sex-related differences in PWTs either at baseline or before drug treatment (Tukey test, p > 0.05). Analysis of PWTs identified a significant interaction of Sex Drug (F(1, 23) = 30.88, p = 0.00001), indicating that mechanical allodynia was attenuated by ICAP in males but not females. Thus, in male rats, post-treatment administration of ICAP increased PWTs at each of the five post-ICAP time points tested compared to the ineffective vehicle control treatment (p = 0.0003, p = 0.006, p = 0.010, p = 0.00004 and p = 0.0393, respectively, Figure 3(c)). In contrast, the spinal administration of ICAP to female animals was without effect when similarly compared to the vehicle treatment group (Figure 3(d)).
Effects of Tat-GluA1(L2-3) in the formalin test and following i.m. acidic saline
We next examined the potential downstream targets of spinal PKCι/λ to nociceptive processing by disrupting the interaction between scaffolding protein p62 and the GluA1 subunit of AMPAR, targets shown to be downstream of PKCι/λ in the hippocampus.23 The p62/GluA1 interaction was disrupted by the spinal administration of cell-permeable peptide Tat-GluA1(L2-3).23 There was no significant change in the biphasic pattern of nociceptive behaviours produced by hind paw formalin injection in either male or female animals pre-treated with Tat-GluA1(L2-3) compared to formalin-induced nociceptive behaviours that occurred following pre-treatment with scrambled-Tat-GluA1(L2-3) (Figure 4(a) and (b)). Thus, no sex differences were found after the inhibition of interactions of p62/GluA1 by Tat-GluA1(L2-3) in the formalin test.
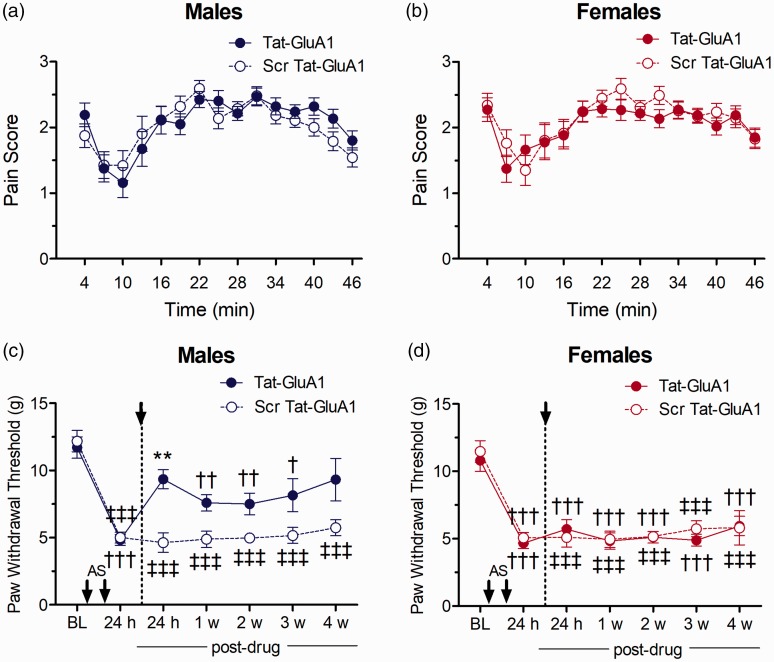
Figure 4.
Effect of Tat-GluA1(L2-3) (p62/GluA1 inhibition) as compared to scrambled peptide on nociceptive responses in the formalin test in (a) male and (b) female rats, or on mechanical allodynia after i.m. acidic saline in (c) males and (d) females (n=6/group). Asterisks indicate significant differences between PWTs following Tat-GluA1(L2-3) and scrambled control peptide treatment (**p<0.01, Tukey test). Daggers indicate differences in PWTs compared to baseline measures for Tat-GluA1(L2-3)-treated animals (†p<0.05, ††p<0.01, †††p<0.001, Dunnett test), while double daggers indicate difference from baseline in Scr-Tat-GluA1(L2-3)-treated animals (‡‡‡p<0.001, Dunnett test).
We next assessed the effects of Tat-GluA1(L2-3) and its control peptide on allodynia in i.m. acidic saline-treated rats. Mechanical allodynia was established 24 h after the second acidic saline injection for both sexes, with a significant reduction in PWTs compared to baseline measures. There were no sex-related differences in PWTs either at baseline or before drug treatment (Tukey test, p > 0.05). Post-treatment of spinal Tat-GluA1(L2-3) resulted in an increase in mean PWTs in males, which were significantly higher than PWTs for rats treated with scr-Tat-GluA1(L2-3) at 24 h after i.t. drug administration (p = 0.0117; Figure 4(c)). In contrast, PWTs in females remained significantly reduced from baseline following treatment with Tat-GluA1(L2-3) and did not differ significantly from those in rats treated with scr-Tat-GluA1(L2-3) at any time point (Figure 4(d)), giving rise to a significant interaction of Sex Drug (F(1, 20) = 20.09, p = 0.00023).
Effects of myr-pep2m in the formalin test and following i.m. acidic saline
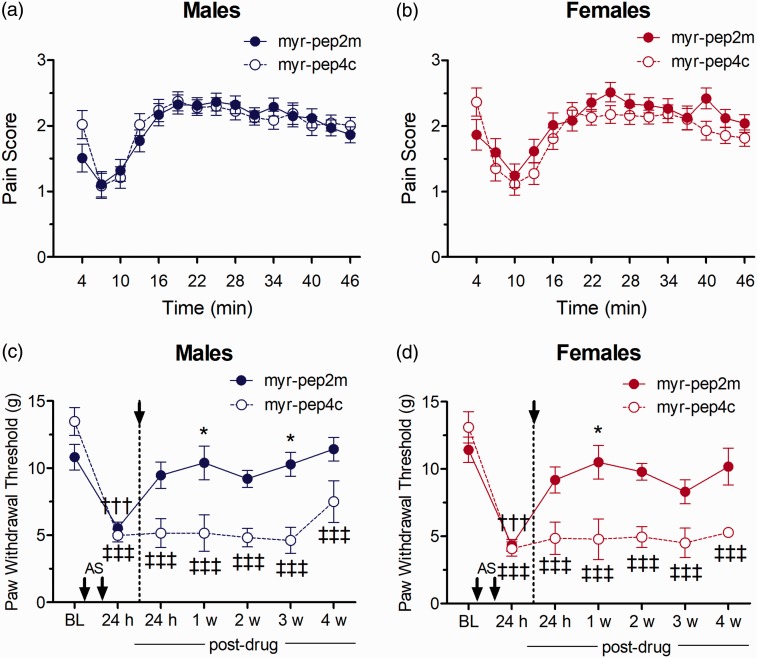
We next examined the downstream targets of spinal PKMζ on nociceptive processing by disrupting the interaction between NSF and the GluA2 subunit of the AMPAR. Previous work, using only male animals, showed that spinal inhibition of NSF/GluA2 prevented the development of mechanical allodynia in a hyperalgesic priming test using interleukin-6/prostaglandin E2 (IL6/PGE2).13 The NSF/GluA2 interaction was disrupted by the cell-permeable peptide myr-pep2m and compared to effects of the inactive control peptide, myr-pep4c.13 In the formalin test, pre-treatment with i.t. myr-pep2m did not significantly alter the biphasic pattern of nociceptive behaviours compared with pre-treatment myr-pep4c, in either sex (Figure 5(a) and (b)).
Figure 5.
Effect of myr-pep2m (NSF/GluA2 inhibition) on nociceptive responses in the formalin test in (a) males and (b) female rats, or on mechanical allodynia following i.m. acidic saline in (c) males and (d) females (n=6/group). Asterisks indicate significant differences between myr-pep2m- and scrambled control peptide-treated rats (*p<0.05, **p<0.01, ***p<0.001, Tukey test). Daggers indicate differences in PWTs compared to baseline measures for myr-pep2m-treated animals (†††p<0.001, Dunnett test), while double daggers indicate difference from baseline in myr-pep4c-treated animals (‡‡‡p<0.001, Dunnett test).
The role of spinal PKMζ substrate NSF/GluA2 was then assessed on the established allodynia produced by i.m. acidic saline, using the inhibitor myr-pep2m. The repeated i.m. injections of acidic saline produced a significant reduction in PWTs from baseline measures in both female and male rats, establishing mechanical allodynia for both sexes. There were no sex differences in PWTs either at baseline or before drug treatment (Tukey test, p > 0.05). Post-treatment spinal administration with myr-pep2m resulted in an increase in PWTs, in both male and female rats at various time points tested (Time Drug interaction: F(1, 17) = 35.2, p = 0.00001). Thus, myr-pep2m-treated male rats displayed PWTs significantly greater than those of myr-pep4c-treated animals at one week (p = 0.0195) and three weeks (p = 0.006) following drug administration (Figure 5(c)), while myr-pep2m-treated females differed from their myr-pep4c-treated counterparts at one week post-treatment (p = 0.0135; Figure 5(d)).
NSF/GluA2 disruption following i.t. CGRP
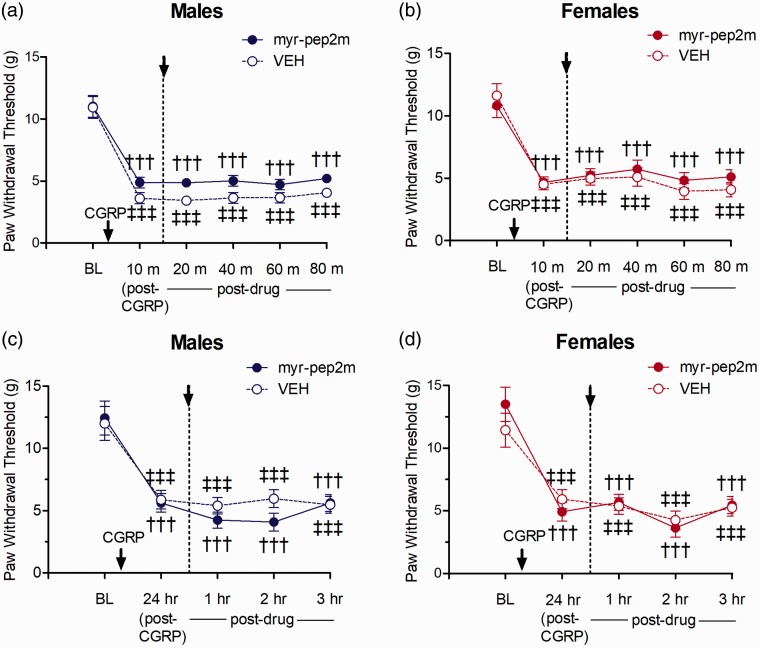
We assessed whether female-specific atypical PKC mechanisms may be driven by CGRP, as spinal administration of a CGRP receptor agonist was previously shown to reverse mechanical allodynia produced by hyperalgesic priming in females, but not males.31 I.t. administration of low dose CGRP30 resulted in a robust acute allodynic effect, with a significant reduction in PWTs from baseline lasting for 90 min following injection in both males and females. Spinally administered myr-pep2m delivered after CGRP-induced allodynia was established did not elevate PWTs across the 90-min testing period for either sex, with no significant differences between the PWTs in female or male rats compared to thresholds in rats treated with the inactive vehicle control. Thus, no sex differences were found for the effects of inhibition of NSF/GluA2 on acute CGRP-induced allodynia (Figure 6(a) and (b)).
Figure 6.
Effect of myr-pep2m (NSF/GluA2 inhibition) on acute mechanical allodynia induced following i.t. CGRP in (a) males and (b) females, or persistent mechanical allodynia induced 24 h following high-dose i.t. CGRP in (c) males and (d) females (n=6/group). Paw withdrawal thresholds did not differ between myr-pep2m- and vehicle control-treated female or male rats for either test.
CGRP: calcitonin gene-related peptide.
A higher dose of i.t. CGRP produced a long-lasting allodynia after administration, with PWTs significantly reduced compared to baseline measures for >24 h. Post-treatment of spinal myr-pep2m again did not increase the PWTs compared to vehicle control, for either males or females. Thus, no sex differences were found for the inhibition of interactions of NSF/GluA2 on persistent CGRP-induced allodynia (Figure 6(c) and (d)).
Sex differences in drug effects on i.m. acidic saline-induced allodynia
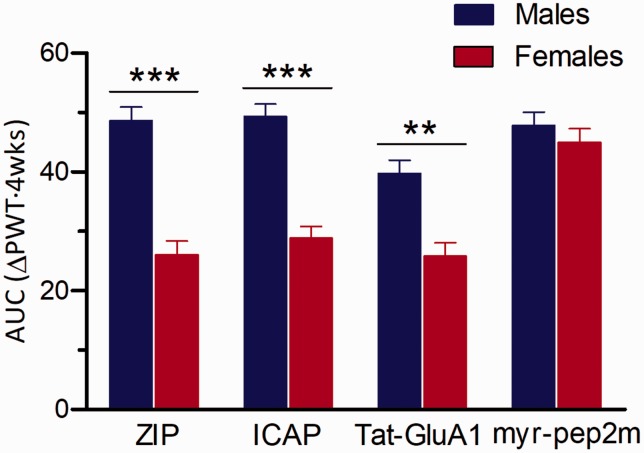
For experiments assessing intramuscular acetic saline-induced allodynia, we also calculated AUCs from the time course data for each drug treatment (ZIP, ICAP, Tat-GluA1(L2-3), myr-pep2m) to directly compare the responses of males and females to inhibition of aPKCs, PKCι λ, p62/GluA1 and NSF/GluA2. Comparison of male and female responses to ZIP, ICAP and Tat-GluA1(2L-3) indicated robust sex differences (p = 0.00013, p = 0.00014, p = 0.002, respectively), while there were no sex-related effects of interactions on allodynia detected after inhibition of NSF/GluA2 with myr-pep2m (p = 0.9871) (Figure 7).
Figure 7.
Male and female responses to each drug following intramuscular acidic saline were compared by analyzing the area under the time-effect curve (AUC). There was a significant Sex Drug interaction (F(3, 43) = 8.005, p = 0.00024). The AUC of male PWTs across the four-week time course was significantly greater than the female AUC for the drugs ZIP, ICAP and TAT-GluA1, while there was no difference between sexes in the response to myr-pep2m. Asterisks indicate significant differences between AUC of male and female (n=6/group) PWTs for each drug (**p<0.01, ***p<0.001, Tukey test).
ICAP: PKCι/λ-antagonist; AUC: area under the curve, PWT: paw-withdrawal threshold.
Discussion
These findings provide novel insights into the contributions of atypical PKC isoforms and their downstream targets in the maintenance of hypersensitivity underlying persistent pain, as well as identify critical sex differences in the mechanisms underlying spinal nociceptive sensitization. Nearly all previous work related to PKMζ and pain has relied on non-specific inhibition of atypical PKCs by ZIP, with some investigations using viral overexpression of PKC/Mζ13 or constitutive genetic ablation.3 This was the first known selective inhibition of the atypical isoform PKCι/λ in spinal nociceptive sensitization, as well as the first assessment of its downstream target p62/GluA1, thus allowing for further examination of spinal nociceptive role of the different atypical PKC isoforms that are inhibited by ZIP.
Non-specific atypical PKC inhibition by ZIP resulted in a male-specific reversal of allodynia induced by repeated muscular injury, consistent with previous work in this same paradigm.3 Interestingly, the hypothesized role of PKCι/λ in the maintenance of spinal nociceptive sensitization in females was not supported by these results, as female rats continued to exhibit allodynia following administration of inhibitors to both PKCι/λ and the downstream target p62/GluA1 in multiple pain tests. These results, in addition to previous work identifying PKMζ-dependent sex differences,3 suggest that persistent hypersensitivity in females is likely to be maintained by factors independent of atypical PKC isoforms. Interestingly, inhibition of the PKMζ downstream target NSF/GluA2 using myr-pep2m reversed allodynia following i.m. acidic saline injury in both males and females. The analgesic effect of disrupting NSF/GluA2 in females is inconsistent with previous pharmacological and genetic manipulations of PKMζ,3 suggesting that in female rats, NSF/GluA2 interactions may be the downstream target of something other than PKMζ.
Notably, the previous research that established the PKMζ-NSF/GluA2 pathway in the hippocampus relied exclusively on male animals and thus did not assess potential sex differences.32,33 However, differential utilization of PKMζ was demonstrated across the sexes in one study of long-term spatial memory, with males showing a significant increase in synaptic PKMζ expression positively correlated with memory retention scores, while females did not.34 Interestingly, and similar to the present findings, despite the sex divergent role of PKMζ for memory retention, both female and male rodents demonstrated a positive correlation between retention test scores and synaptic GluA2 expression.34 Based on the findings of this present study, the common interaction between NSF and the GluA2 subunit of AMPAR appears to be driven by divergent upstream mechanisms in males and females. Further investigation into female specific mechanisms is necessary to delineate sex-specific pathways, as well as to identify the point at which a mechanistic switch occurs; reflecting a divergence from a shared molecular pathway between the two sexes underlying sex-specific signalling.35 Future studies focused on the interactions of NSF, and potential female-specific upstream mediators could assist to identify female-specific targets involved in the maintenance of centrally-mediated persistent hypersensitivity.
CGRP signalling was hypothesized to be a potential alternative upstream mediator of NSF/GluA2 that might be female specific. However, the lack of an anti-allodynic effect of myr-pep2m following i.t. CGRP (either acutely or at 24 h) did not support this. Brain-derived neurotrophic factor has been shown to stimulate PKMζ phosphorylation and the synthesis of PKMζ and PKCι/λ through the activation of PDK1/AKT/mTOR signalling at both spinal and cortical synapses, though notably only male animals have been assessed.14 It is of interest to determine if this regulation is similar across the sexes, given the demonstrated sex differences established by this study and similar work by Nasir et al.3 Additionally, hyperalgesic priming tests with i.m. acidic saline, using only female mice, supports the notion that priming is induced by a glutamate-/PKC-/ERK-/AMPAR-dependent pathway leading to synaptic plasticity at the spinal dorsal horn.36 Further investigation into possible sex differences in PKC modulation of hyperalgesic priming would be of interest.
Inhibition of both PKCι/λ and the downstream target p62/GluA1 resulted in analgesic effects in males after i.m. acetic saline. This is consistent with the effects of inhibition of PKMζ with ZIP or constitutive PRKCZ knockout on nociceptive behaviours induced by i.m. acidic saline,3 suggesting that both atypical PKC isoforms PKCι/λ and PKMζ may contribute to the maintenance of spinal nociceptive sensitization in males. Moreover, the analgesic effect of PKCι/λ inhibition is of particular interest as it highlights a potential mechanistic difference between the maintenance of spinal nociceptive plasticity and hippocampal plasticity, as in contrast to its effects on nociception, PKCι/λ was only shown to play a compensatory role in hippocampal late-LTP following genetic ablation of PKMζ and not in wild-type animals.24 Identifying distinctions between spinal and cortical mechanisms would be helpful for future analgesic drug development by allowing for the discovery of agents that produce analgesia but do not disrupt memory formation or cortical function.7,37 Analgesic drug development has thus far been limited by the underlying mechanistic similarities between central sensitization and hippocampal plasticity and LTP. For example, NMDA receptor antagonists such as ketamine effectively reduce pain hypersensitivity, but severely impact cortical functioning.37 Thus, due to the differential effects of its inhibitor on hippocampal LTP and spinal nociceptive sensitization, PKCι/λ may be a potential target of interest for future drug development for males.
There were demonstrated test-specific effects across the different pharmacological manipulations used in this study. Drugs were found to have no effect in the formalin test, while they demonstrated robust reversals of mechanical allodynia following i.m. acidic saline. The formalin test involves peripheral inflammation of the ipsilateral hind paw following formalin injection. Previous work has examined the influence of the nociceptive and inflammatory effects associated with varied concentrations of formalin on the effectiveness of drugs to reduce formalin pain by targeting central sensitization.38,39 Thus, it has been proposed that the peripheral inflammation and accompanying peripheral signalling to the spinal cord may continuously contribute to pain perception, masking any effects of reduced central sensitization, as previously demonstrated by reduced ZIP effects with increasing concentrations of formalin.3 The lack of effect of PKCι/λ, p62/GluA1 or NSF/GluA2 inhibition in the formalin pain test is consistent with the previously demonstrated lack of analgesic effect of ZIP after chronic constriction injury of the sciatic nerve,12,16 spinal nerve ligation,17 spared nerve injury3 or plantar incision.18 In contrast to the formalin test, and these other pain tests, i.m. acidic saline is used as a measure of remote allodynia; pain hypersensitivity which differs in location of expression from the site of tissue injury (i.e., plantar surface of the hind paw and gastrocnemius muscle, respectively), thus allowing for a behavioural investigation of centrally-mediated mechanisms spatially separated from ongoing noxious peripheral input. The findings presented here are consistent with ZIP effects after hyperalgesic priming involving IL6/PGE2,13 brain-derived neurotrophic factor/PGE2,14 IL6/plantar incision,15 (in which priming and testing are temporally separated) and repeated injections of i.pl. and intracolonic capsaicin (which reflect both temporal and spatial separation, respectively).3 Thus, the reported differences in inhibition of atypical PKCs and downstream targets across the two pain tests (formalin, i.m. acidic saline) indicate the influence of continued afferent input on the modulation of central sensitization. These findings provide further support for the notion that analgesia produced by inhibition of PKMζ is masked by ongoing peripheral input to the spinal cord,3 suggesting that both atypical PKC isoforms contribute mainly to the maintenance of spinal nociceptive sensitization.
Investigation into the role of PKMζ in nociceptive sensitization has not been limited to the level of the spinal cord, with ZIP administration to the anterior cingulate cortex (ACC) establishing a key role of cortical atypical PKCs in spontaneous pain evoked by nerve injury.40 Interestingly, spinal and cortical pain mechanisms related to atypical PKCs appear to differ in regard to the influence of peripheral input, as ZIP administration to the ACC is able to alleviate neuropathic pain,17,40 despite a number of studies demonstrating no effect after i.t. injections.3,12,16 As of yet, experiments examining pain plasticity in the ACC have not examined the contribution of PKCι/λ, and there has been limited investigation into female-specific mechanisms. However, recent translational work investigating neural pain memory demonstrated that the male-specific conditioned pain hypersensitivity was abolished by both i.t. and intra-cerebral injections of ZIP, interestingly demonstrating the influence of atypical PKCs on neuronal pain memory at both the spinal and cortical levels. Additionally, these authors found that the context-dependent pain hypersensitivity in male mice was eliminated by castration or by pharmacological blockade of the hypothalamic-pituitary-adrenal axis, indicating the influences of testosterone and stress, respectively.41 Further study into how pain memories are generated and able to maintain hypersensitivities, particularly across the sexes, is a necessary and exciting new area for future pain research.
Overall, our results demonstrate novel behavioural evidence for the male-specific role for PKCι/λ and its downstream target p62/GLuA1, in addition to PKMζ, in pain states for which central sensitization is a key component. The sexually divergent pathways appear to converge at the interaction between NSF and the GluA2 subunit of the AMPA receptor. While the NSF/GluA2 interaction is thought to be downstream of PKMζ in males, these findings and previous PKMζ-dependent sex differences3 suggest that females may rely on a thus far unidentified factor, independent of atypical PKCs, for the maintenance of persistent pain states. These behavioural findings will inform future investigation of basic mechanisms related to the maintenance of central plasticity, a critical step towards identifying targets for analgesic drug development.
Acknowledgments
The authors wish to thank Dr. Robert Farese, Department of Internal Medicine, James A Haley Veterans Hospital, University of South Florida, Tampa, FL, for supplying the ICAP.
Author Contributions
TJC and NG designed the study; NG and AL conducted behavioural experiments; NG and AL performed statistical analysis; NG prepared figures; NG wrote the paper and TJC and AL supervised experiments and edited the paper.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (RGPIN/05605), the Canadian Institutes of Health Research (MOP-119279) and the Louise and Alan Edwards Foundation. NG was supported by a Fredrick Banting and Charles Best Canada Graduate Scholarship-Master’s (CGS-M).
References
- 1.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 2003; 26: 696–705. [DOI] [PubMed] [Google Scholar]
- 2.Price TJ, Inyang KE. Commonalities between pain and memory mechanisms and their meaning for understanding chronic pain. Prog Mol Biol Transl Sci 2015; 131: 409–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nasir H, Mahboubi H, Gyawali S, Ding S, Mickeviciute A, Ragavendran JV, Laferrière A, Stochaj U, Coderre TJ. Consistent sex-dependent effects of PKM zeta gene ablation and pharmacological inhibition on the maintenance of referred pain. Mol Pain 2016; 12: 1744806916675347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang L, Wu J, Lin Q, Willis WD. Calcium–calmodulin-dependent protein kinase II contributes to spinal cord central sensitization. J Neurosci 2002; 22: 4196–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci 1999; 2: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 6.Sluka KA, Willis WD. The effects of G-protein and protein kinase inhibitors on the behavioral responses of rats to intradermal injection of capsaicin. Pain 1997; 71: 165–178. [DOI] [PubMed] [Google Scholar]
- 7.Price TJ, Ghosh S. ZIPping to pain relief: the role (or not) of PKMζ in chronic pain. Mol Pain 2013; 9: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling DSF, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mζ is necessary and sufficient for LTP maintenance. Nat Neurosci 2002; 5: 295–296. [DOI] [PubMed] [Google Scholar]
- 9.Serrano P, Yao Y, Sacktor TC. Persistent phosphorylation by protein kinase Mζ maintains late-phase long-term potentiation. J Neurosci 2005; 25: 1979–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science 2006; 313: 1141–1144. [DOI] [PubMed] [Google Scholar]
- 11.Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKMζ. Science 2007; 317: 951–953. [DOI] [PubMed] [Google Scholar]
- 12.Laferrière A, Pitcher MH, Haldane A, Huang Y, Cornea V, Kumar N, Sacktor TC, Cervero F, Coderre TJ. . PKM zeta is essential for spinal plasticity underlying the maintenance of persistent pain. Mol Pain 2011; 7: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asiedu MN, Tillu DV, Melemedjian OK, Shy A, Sanoja R, Bodell B, Ghosh S, Porreca F, Price TJ. Spinal protein kinase M zeta underlies the maintenance mechanism of persistent nociceptive sensitization. J Neurosci 2011; 31: 6646–6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melemedjian OK, Tillu DV, Asiedu MN, Mandell EK, Moy JK, Blute VM, Taylor CJ, Ghosh S, Price TJ. BDNF regulates atypical PKC at spinal synapses to initiate and maintain centralized chronic pain state. Mol Pain 2013; 9: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.An K, Zhen C, Liu ZH, Zhao Q, Liu HP, Zhong XL, Huang WQ. Spinal protein kinase M zeta contributes to the maintenance of peripheral inflammation-primed persistent nociceptive sensitization after plantar incision. Eur J Pain 2015; 19: 39–47. [DOI] [PubMed] [Google Scholar]
- 16.Marchand F, D’Mello R, Yip PK, Calvo M, Muller E, Pezet S, Dickenson AH, McMahon SB. Specific involvement of atypical PKCζ/PKMζ in spinal persistent nociceptive processing following peripheral inflammation in rat. Mol Pain 2011; 7: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King T, Qu C, Okun A, Melemedjian OK, Mandell EK, Maskaykina IY, Navratilova E, Dussor GO, Ghosh S, Price TJ, Porreca F. Contribution of PKM zeta-dependent and independent amplification to components of experimental neuropathic pain. Pain 2012; 153: 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, Li C, Guo Y, Xing Y, Tao F. PKMζ is not required for development of postsurgical pain. Molecular Neurobiol 2018; 55: 2397–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parada C, Reichling D, Levine J. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCɛ second messenger pathways. Pain 2005; 113: 185–190. [DOI] [PubMed] [Google Scholar]
- 20.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci 2009; 32: 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee AM, Kanter BR, Wang D, Lim JP, Zou ME, Qiu C, McMahon T, Dadgar J, Fischbach-Weiss SC, Messing RO. Prkcz null mice show normal learning and memory. Nature 2013; 493: 416–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volk LJ, Bachman JL, Johnson R, Yu Y, Huganir RL. PKM-zeta is not required for hippocampal synaptic plasticity, learning and memory. Nature 2013; 493: 420–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren SQ, Yan JZ, Zhang XY, Bu YF, Pan WW, Yao W, Tian T, Lu W. PKC lambda is critical in AMPA receptor phosphorylation and synaptic incorporation during LTP. EMBO J 2013; 32: 1365–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsokas P, Hsieh C, Yao Y, Lesburgueres E, Wallace EJ, Tcherepanov A, Jothianandan D, Hartley BR, Pan L, Rivard B, Farese RV, Sanjan M, Bergold PJ, Hernandez AI, Cottrell JE, Shouval HZ, Fenton AA, Sactor TC. Compensation for PKMzeta in long-term potentiation and spatial long-term memory in mutant mice. Elife 2016; 5: e14846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Sheng T, Ren S, Tian T, Lu W. Distinct roles of PKCι/λ and PKMζ in the initiation and maintenance of hippocampal long-term potentiation and memory. Cell Rep 2016; 16: 1954–1961. [DOI] [PubMed] [Google Scholar]
- 26.Sheng T, Wang S, Qian D, Gao J, Ohno S, Lu W. Learning-induced suboptimal compensation for PKCι/λ function in mutant mice. Cereb Cortex 2017; 27: 3284–3293. [DOI] [PubMed] [Google Scholar]
- 27.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 28.Sluka K, Kalra A, Moore S. Unilateral intramuscular injections of acidic saline produce a bilateral, long‐lasting hyperalgesia. Muscle Nerve 2001; 24: 37–46. [DOI] [PubMed] [Google Scholar]
- 29.Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977; 4: 161–174. [DOI] [PubMed] [Google Scholar]
- 30.Sun RQ, Tu YJ, Lawand NB, Yan JY, Lin Q, Willis WD. Calcitonin gene-related peptide receptor activation produces PKA-and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol 2004; 92: 2859–2866. [DOI] [PubMed] [Google Scholar]
- 31.Paige C, Mejia G, Maruthy GB, Price T. Microglia promote pain plasticity in males while CGRP promotes pain plasticity in females in the hyperalgesic priming model. J Pain 2017; 18: S9. [Google Scholar]
- 32.Yao Y, Kelly MT, Sajikumar S, Serrano P, Tian D, Bergold PJ, Frey JU, Sacktor TC. PKM zeta maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J Neurosci 2008; 28: 7820–7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. PKM zeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci 2010; 13: 630–634. [DOI] [PubMed] [Google Scholar]
- 34.Sebastian V, Vergel T, Baig R, Schrott LM, Serrano PA. PKM zeta differentially utilized between sexes for remote long-term spatial memory. PLoS One 2013; 8: e81121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mogil JS. Sex-based divergence of mechanisms underlying pain and pain inhibition. Curr Opin Behav Sci 2018; 23: 113–117. [Google Scholar]
- 36.Chen W, Chang Y, Chen Y, Cheng S, Chen C. Spinal protein kinase C/extracellular signal–regulated kinase signal pathway mediates hyperalgesia priming. Pain 2018; 159: 907–918. [DOI] [PubMed] [Google Scholar]
- 37.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011; 152: S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yashpal K, Katz J, Coderre TJ. Effects of preemtive or postinjury intrathecal local anesthesia on persistent nociceptive responses in rats: confounding influences of peripheral inflammation and the general anesthetic regimen. Anesthesiology 1996; 84: 1119–1128. [DOI] [PubMed] [Google Scholar]
- 39.Yashpal K, Coderre TJ. Influence of formalin concentration on the antinoceptive effects of anti-inflammatory drugs in the formalin test in rats: separate mechanisms underlying the nociceptive effects of low- and high-concentration formalin. Eur J Pain 1998; 2: 63–68. [DOI] [PubMed] [Google Scholar]
- 40.Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, Shim J, Lee K, Collingridge GL, Kaang BK, Zhuo M. Alleviating neuropathic pain hypersensitivity by inhibiting PKM zeta in the anterior cingulate cortex. Science 2010; 330: 1400–1404. [DOI] [PubMed] [Google Scholar]
- 41.Martin LJ, Acland EL, Cho C, Gandhi W, Chen D, Corley E, Kadoura B, Levy T, Mirali S, Tohyama S, Khan S, MacIntyre LC, Carlson EN, Schweinhardt P, Mogil JS. Male-specific conditioned pain hypersensitivity in mice and humans. Curr Biol 2019; 29: 192–201. [DOI] [PubMed] [Google Scholar]