Abstract
Objective
To assess the status of malaria prevalence in one of the malaria endemic areas of Ethiopia.
Results
A 10-year report of malaria cases were obtained from Asendabo Health Center, Jimma zone, Southwest Ethiopia. Following a retrospective study design, data of 68, 421 febrile patients diagnosed and treated in the health center were included in the study. The year with the highest prevalence rate (34.9%) was 2010, whereas the lowest was 2016 (0.62%). The number of diagnosed malaria cases from September to November were significantly higher (P = 0.023, n = 6336, 46.5%) than in other months. Plasmodium falciparum (52.1%, n = 7087) and Plasmodium vivax (44.2%, n = 6009) were the two principal plasmodium species accountable for malaria infections in the study area. The current study is a supportive evidence for the reduction of malaria prevalence in malaria endemic areas of Ethiopia.
Electronic supplementary material
The online version of this article (10.1186/s13104-019-4329-6) contains supplementary material, which is available to authorized users.
Keywords: Malaria, Prevalence, P. falciparum, P. vivax, Seasonal variation
Introduction
Malaria is one of the top public health concerns of the nations in tropical and sub-tropical regions of the world. It is a major cause of morbidity and mortality in Africa [1]. Reports in 2005, showed that global estimate of deaths that occurred annually from malaria was 1–3 million [2]. However, a recent report of World Health Organization (WHO) showed a significant reduction of malaria associated deaths to 584,000 globally in 2013 [3]. Other reports by WHO has also indicated the reduction of malaria-related deaths among African children since 2000 [4].
In Ethiopia malaria is still one of the major health problems in some parts of the country. According to the Federal Ministry of Health (FMoH) report, about 60% of the country’s populations live in malaria endemic areas [5]. To create malaria-free nation, government has set a big goal of eliminating malaria by 2020 [5]. To achieve the set goal, all concerned bodies in the country have shown a strong commitment. Hence, the country has been able to achieved about 50% overall malaria reduction goal by 2015 [6]. This remarkable achievement was attained mainly due to the implementation of intensive interventional strategies such as indoor residual spraying, use of bed nets, and combination chemotherapy [7]. However, in some endemic areas of the same country, this reduction has not yet been achieved and even it is a major cause of illness and death [8]. One of the regions that have so far achieved remarkable malaria burden reduction is Oromia [9]. Jimma zone is one of malaria-endemic areas in the region and Asendabo is one of malaria endemic districts found in the zone (https://en.wikipedia.org/wiki/Jimma_Zone). In the district, intensive malaria interventional approaches have been executed for a long time. Thus, the current study was designed to assess the status of malaria prevalence in this district and draw the experience that could be shared by other similar districts.
Main text
Methods
Study area
The study was conducted in Asendabo district, located at 303 km southwest of Ethiopia. This district is one of the malaria endemic areas in the zone and received special attention from national and regional governments, NGOs and public institutions such as Jimma University, Center for tropical and infectious disease on malaria prevention and control campaigns [personal communication]. The district has only one health center that serves about a population of 33,981 (unpublished data from zonal health office). Malaria is one of the major health problems of the district. The two main plasmodium species: P. vivax and P. falciparum are responsible for malaria infection and Anopheles arabiensis is known as principal vector transmitting malaria in the district.
Study design and data collection process
A retrospective study was conducted to determine the prevalence of malaria by reviewing febrile patients’ medical record at Asendabo Health Center from September 2007 to August, 2017 (10 year period). Patients were diagnosed and treated following a standard operating procedure for malaria diagnosis. Peripheral blood smear examination was used to confirm malaria infection as per the recommendation of WHO. All the variables recoded and documented for each patients such as Age in range, sex, blood film status (+ve and −ve), plasmodium species (P. falciparum, P. vivax and mixed infection), and data of diagnosis on the weekly malaria reporting format were considered in the study.
Data analysis
Data was checked for correctness, and analyzed using SPSS (Armonk, NY: IBM Corp) version 20.0 software. Descriptive statistical tests were used for analysis of malaria prevalence, seasonal variation and demographic data. A One sample T-test and Relative Risk test were statistical tools used to analyze differences between means of variables and to show relative risk of different groups to malaria respectively. Significance level was considered at confidence interval (CI) of 95%.
Results
Trends of malaria prevalence
In the current study data of 68,421 febrile patients diagnosed and treated in the health center over the 10 years were considered. About 13, 624 of them were malaria positive. This showed an aggregate malaria prevalence of 20.7% (95% CI 20.37–20.99) among febrile patients. From the positive patients, 52.5% (n = 7138, 95% CI 51.32–53.65) were males and 47.5% (n = 6457, 95% CI 46.28 to 48.73) were females. Prevalence of malaria among biologically risked group, children < 5 years, was 24.4% (n = 3315, 95% CI 22.91–25.86) (Table 1). Although the proportion of malaria positive children was large, their relative risk to acquire the infection compared to the adult’s (RR = 0.77, 95% CI 0.75–0.803) was significantly low.
Table 1.
Trend showing 10 years malaria prevalence in Asendabo Health Center (2007–2016)
| Year | Total examined | Malaria positive | Malaria prevalence | Patients in sex | <5 years (%) | |
|---|---|---|---|---|---|---|
| Male (%) | Female (%) | |||||
| 2007 | 6497 | 1814 | 27.9 | 953 (52.5) | 861 (47.5) | 392 (21.6) |
| 2008 | 6506 | 1802 | 27.7 | 910 (50.5) | 892 (49.5) | 375 (20.8) |
| 2009 | 7266 | 2104 | 28.9 | 1058 (50.3) | 1046 (49.7) | 617 (29.3) |
| 2010 | 14,235 | 4963 | 34.9 | 2670 (53.8) | 2293 (46.2) | 1205 (24.3) |
| 2011 | 9095 | 1916 | 21.1 | 1020 (53.2) | 896 (46.8) | 452 (23.6) |
| 2012 | 7218 | 770 | 10.7 | 401 (52.1) | 369 (47.9) | 211 (27.4) |
| 2013 | 4578 | 137 | 2.99 | 76 (55.5) | 61 (44.5) | 35 (25.5) |
| 2014 | 3654 | 38 | 1.04 | 21 (55.3) | 17 (44.7) | 6 (15.8) |
| 2015 | 3879 | 33 | 0.85 | 15 (45.5) | 18 (54.5) | 13 (39.4) |
| 2016 | 2874 | 18 | 0.62 | 14 (77.7) | 4 (22.2) | 9 (50) |
| 2017 | 2619 | 29 | 1.1 | 18 (62.1) | 11 (37.9) | 10 (34.5) |
| Total | 65,802 | 13,595 | 20.7 | 7138 (52.5) | 6457 (47.5) | 3315 (24.4) |
Although the cumulative 10 years prevalence of malaria in febrile patients shown high, the trend in the study area has a declining pattern, from 27.9% in 2007 to 0.62% in 2016. The highest malaria prevalence among febrile patients (34.9%, n = 4963) was documented in the year 2010 and followed by 2009 (29.4%, n = 2104). The recent year’s assessment showed that, starting from 2013 to 2016, the prevalence was drastically reduced from two digits to 0.6% (1.375 averages) (Table 1). In 2016/2017, from September, 2016 to August, 2017, a total of 2619 febrile cases were examined for malaria infection. Only 29 (1.1%) of them were malaria positive. This showed that the declining pattern was persistent with slight increment from 0.6% (18/2874) in 2015/16 to 1.1% (29/2619) in 2016/2017, but not significantly different (P = 0.056). Majority of the positive cases (n = 17, 58.6%) were registered in a month (August). Among the total 29 positive patients observed, n = 10 were children < 5 years. The two Plasmodium species were accountable for malaria infections in the study area. About 52.1% (n = 7087) and 44.2% (n = 6508) malaria cases were infected with Plasmodium falciparum and P. vivax respectively. The remaining 7.7% were due to mixed (P. falciparum and P. vivax) infection (Fig. 1).
Fig. 1.
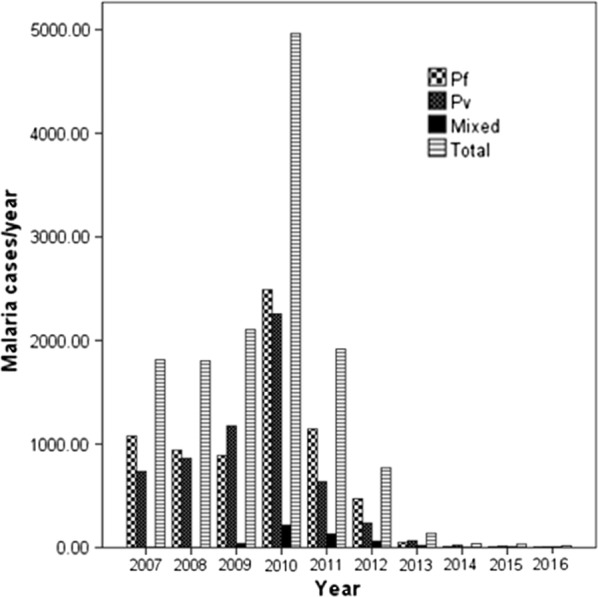
Plasmodium species accountable for malaria infection in Asendabo Health Center (2007–2016). Pf: Plasmodium falciparum; Pv: Plasmodium vivax; Mixed: infection with both P.f and P.v
Seasonal variation and prevalence of malaria
From the analysis made to assess role of seasonal variations on the prevalence of malaria among febrile patients, it was observed that Autumn season (from September to November in Ethiopia) was found as malaria peak season in the study area. In other seasons such as Spring, Winter and Summer, the prevalence pattern seems similar. Number of malaria diagnosed patients in Autumn (46.5%, n = 6336) was significantly higher (P = 0.023) than other seasons. Seasonal variation showed effects on the type of plasmodium parasite infection. While P. falciparum and P. vivax were the two dominant parasite, prevalence of P. vivax among febrile patients was significantly higher (P = 0.042) compared to P. falciparum during Winter season (December to February in Ethiopia) than other seasons (Additional file 1).
Discussion
In the current study the aggregate 10 years malaria prevalence observed was 20.7%. The prevalence rate was varies from 34.9% prevalence in 2010 to 0.62% in 2016. Similar to the situation in other developing countries, malaria was the major public health problem in the study area. However, recently due to a strong commitment shown by concerned bodies, the prevalence of malaria is radically reducing in most parts of the country [6]. Thus, the observed significant reduction in malaria prevalence may suggest the effectiveness of the interventional strategies adopted in the study area.
While the decreasing pattern of malaria prevalence throughout the 10 year was consistent, the fact that slight increment observed in the recent year (2016/17) has indication of possibility of malaria resurgence in the study area. Although it is possible to achieve remarkable malaria prevalence reduction, complete elimination of the infection is impossible [10, 11]. This could be due to the presence of persistent residual infection transmission and the biological nature of the parasite [12]. Thus, close monitoring and surveillance of the interventional strategies and the infection pattern is very important in such locality.
One of the determinant factors for malaria transmission is seasonal variation. Temperature and humidity are variables that govern plasmodium parasite growth in the vector body, and the vector development in the environment. Thus, optimum environmental condition is very important for the disease to transmit. Autumn season (after heavy rain) was found to be a malaria peak transmission season in the study area [13]. This finding is in agreement with a report of Jamil and Khan [14] from Pakistani and Woyessa et al. [15] from Butajira-Ethiopia, where prevalence of P. falciparum malaria reached its highest frequency after heavy rain.
One of the factors that might have contributed to the successful malaria reduction in the country is, direct involvement of health extension workers (HEWs) in malaria control and prevention campaign [16]. Their major role is enhancing awareness of the community towards malaria infection, its transmission, and on how to use of different prevention methods in malaria endemic areas [16, 17]. These workers spend 75% of their time visiting families in their homes and performing outreach activities in the community and distribute artemisinin-based combination therapy (ACT) to the needy community [18]. Thus, presence of HEWs is one of the important factors for early observation and reporting of malaria cases, for further diagnosis at the nearby health facilities, and to get treatment with appropriate antimalarial drugs [19]. This could be considered as best practice to be shared by other similar people living in malaria endemic areas in the country and beyond, with similar set-up. In general the use of bed net, IRS, combination therapy and contribution of different stakeholders including HEW might have a great role for the declining of malaria infection in the study area [20].
Conclusion
The current study provides supportive evidence for the reduction of malaria prevalence in Jimma zone, Asendabo district. This could serve as a sign for the rise in commitment of all stakeholders to eliminate malaria from the country. The observed declining of malaria prevalence among febrile patients in this study is a promising outcome which could be cascaded to other malaria endemic areas.
Limitation
This study was delimited to only one district. More concrete evidences could have been generated had it been included more malaria-endemic areas in the zone. In addition, only those patients seeking medication and visited the health center during the study period were included in the study. Data of other suspected malaria patients treated at home or used other traditional medication was not included in the study.
Additional file
Additional file 1: Figure S1. Prevalence of plasmodium species with respect to seasonal variation at Asendabo Health Center, (January, 2007–August, 2016).
Acknowledgements
The authors would like to thank officials of Asendabo Health Center for provision of the data and the study participants for their willingness to participate in the study and genuine response.
Abbreviations
- ACT
artemisinin combination therapy
- DDT
dichlorodiphenyltrichloroethane
- FDRE
Federal Democratic Republic of Ethiopia
- HEW
health extension workers
- IRS
indoor residual spraying
- LLITN
long-lasting insecticide treated nets
- UNICEF
United Nations Children’s Fund
- WHO
World Health Organization
Authors’ contributions
TK and AJ were involved in the designing of this study, data collection, analysis and write-up. TK also involved in the supervision of the research. All authors read and approved the final manuscript.
Funding
This work was financially supported by Jimma University. This funding body had no role in the design, collection, data analysis, interpretation and reporting of data or in writing the manuscript.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the manuscript.
Ethics approval and consent to participate
The study was ethically approved by Research and Ethical Review Board of College of Natural Sciences, Jimma University. All data were generated based on the official permission obtained from concerned offices. Written or verbal consent from participants was not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abdurazak Jemal, Email: jemalabdurazak6@gmail.com.
Tsige Ketema, Email: tsigeketema@gmail.com.
References
- 1.WHO. Malaria control today, 2005. 2005. http://www.who.int/malaria/docs/malaria_control_today_2005.pdf. Accessed 15 Apr 2017.
- 2.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434(7030):214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . World malaria report. Geneva: World Health Organization; 2015. [Google Scholar]
- 4.WHO. World malaria report 2014. Geneva: World Health Organization; 2014. http://www.who.int/malaria/publications/world_malaria_report_2014/report/en. Accessed 3 Sept 2018.
- 5.FMoH . National malaria program monitoring and evaluation plan 2014–2020. Addis Ababa: Federal Democratic Republic of Ethiopia Ministry of Health; 2014. [Google Scholar]
- 6.Deribew A, Dejene T, Kebede B, Assefa G, Adama Y, Misganaw A, et al. Incidence, prevalence and mortality rates of malaria in Ethiopia from 1990 to 2015: analysis of the global burden of diseases 2015. Malar J. 2017;16(1):271. doi: 10.1186/s12936-017-1919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jima D, Medhin A. Malaria prevention and control in Ethiopia: progress and prospects. Fed Democr Repub Ethiop Minist Health Q Health Bull. 2008;1:10–18. [Google Scholar]
- 8.Geleta G, Ketema T. Severe malaria associated with Plasmodium falciparum and P. vivax among children in Pawe Hospital, Northwest Ethiopia. Malaria Res Treat. 2016;56:67. doi: 10.1155/2016/1240962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olana D, Chibsa S, Teshome D, Mekasha A, Graves PM, Reithinger R. Malaria, Oromia regional state, Ethiopia, 2001–2006. Emerg Infect Dis. 2011;17(7):1336–1337. doi: 10.3201/eid1707.100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J. 2014;13:330. doi: 10.1186/1475-2875-13-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith DL, Cohen JM, Chiyaka C, Johnston G, Gething PW, Gosling R, Buckee CO, Laxminarayan R, Hay SI, Tatem AJ. A sticky situation: the unexpected stability of malaria elimination. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120145. doi: 10.1098/rstb.2012.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Killeen GF, Seyoum A, Sikaala CH, Zomboko AS, Gimnig JE, Govella NJ, White MT. Eliminating malaria vectors. Parasites Vectors. 2013;6:172. doi: 10.1186/1756-3305-6-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiwanitkit V, Suyaphun A. Seasonal variation in the prevalence of Plasmodium vivax malarial infection: an observation in northern Thailand. Medscape Gen Med. 2005;7(2):7. [PMC free article] [PubMed] [Google Scholar]
- 14.Jamil S, Khan MN. Seasonal variations of vivax and falciparum malaria: an observation at a tertiary care hospital. J Ayub Med Coll Abbottabad. 2012;24(1):93–95. [PubMed] [Google Scholar]
- 15.Woyessa A, Deressa W, Ali A, Lindtjorn B. Prevalence of malaria infection in Butajira area, south-central Ethiopia. Malar J. 2012;11:84. doi: 10.1186/1475-2875-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teklehaimanot H, Teklehaimanot A. Human resource development for a community-based health extension program: a case study from Ethiopia. Hum Resour Health. 2013;11:39. doi: 10.1186/1478-4491-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medhanyie A, Spigt M, Kifle Y, Schaay N, Sanders D, Blanco R, GeertJan D, Berhane Y. The role of health extension workers in improving utilization of maternal health services in rural areas in Ethiopia: a cross sectional study. BMC Health Serv Res. 2012;12:352. doi: 10.1186/1472-6963-12-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sebhatu A. The implementation of Ethiopia’s Health Extension Program, Ethiopia Good Practice: HEP; 2008. p. 2–5.
- 19.Birhanu Z, Abebe L, Sudhakar M, Dissanayake G, Yihdego YY-E, Alemayehu G, et al. Malaria related perceptions, care seeking after onset of fever and anti-malarial drug use in malaria endemic settings of southwest Ethiopia. PLoS ONE. 2016;11(8):e0160234. doi: 10.1371/journal.pone.0160234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gemechu T, Samuel A, Yewhalaw D. Ten years trend analysis of malaria prevalence in relation to climatic variables in Sibu Sire District, East Wollega Zone, Oromia regional State, Western Ethiopia: a retrospective study. Sci Technol Arts Res J. 2015;4(4):99–105. doi: 10.4314/star.v4i4.14. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Prevalence of plasmodium species with respect to seasonal variation at Asendabo Health Center, (January, 2007–August, 2016).
Data Availability Statement
The datasets supporting the conclusions of this article are included within the manuscript.


