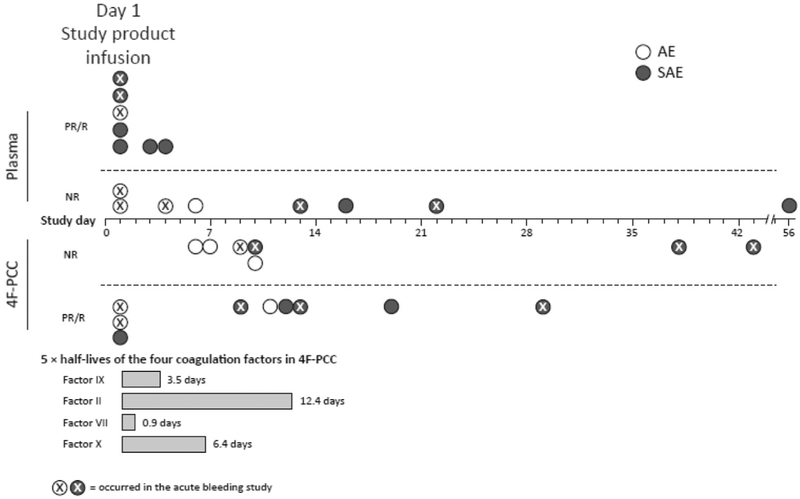
Figure.
Thromboembolic events over time. Three SAEs were not confirmed as a thromboembolic events by the SAB; therefore, the SAB did not assess causality for these events (plasma group: PE [day 16]; 4F-PCC group: thrombosis [day 12] and MI [day 38]); causality indicated as assessed by investigators. AE, Adverse event; PR, possibly or probably related to the study product as assessed by investigators (for adverse events) or SAB (for SAEs); R, related to study product as assessed by investigators (for adverse events) or SAB (for SAEs); NR, not related to study product.