Figure 4: Valine has three distinct rotamer states.
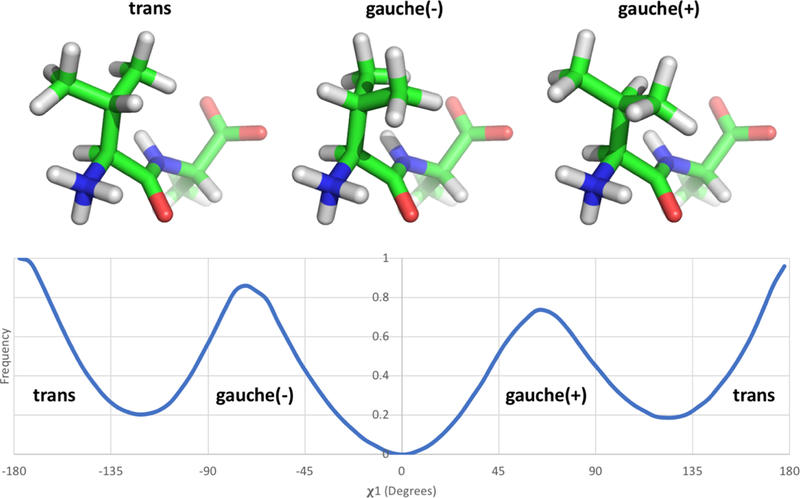
The valine-alanine dipeptide structure is represented here with the N-terminus and valine sidechain positioned at the front and the C-terminus and alanine sidechain at the posterior. The three structures show the valine sidechain χ1 rotamer in each of its three states [trans, gauche(−), and gauche(+)]. The bottom panel shows the frequency of occurrence of the different states indicated above from simulations in solution; the data shown here is based on umbrella sampling of the valine-alanine peptide which is described and discussed further in Section 3.2 and Figure 5. While the qualitative rotameric preferences of valine are expected to remain consistent whether the sidechain is in solution or in a binding site, the actual frequency of each state is likely to vary substantially depending on its surroundings.
