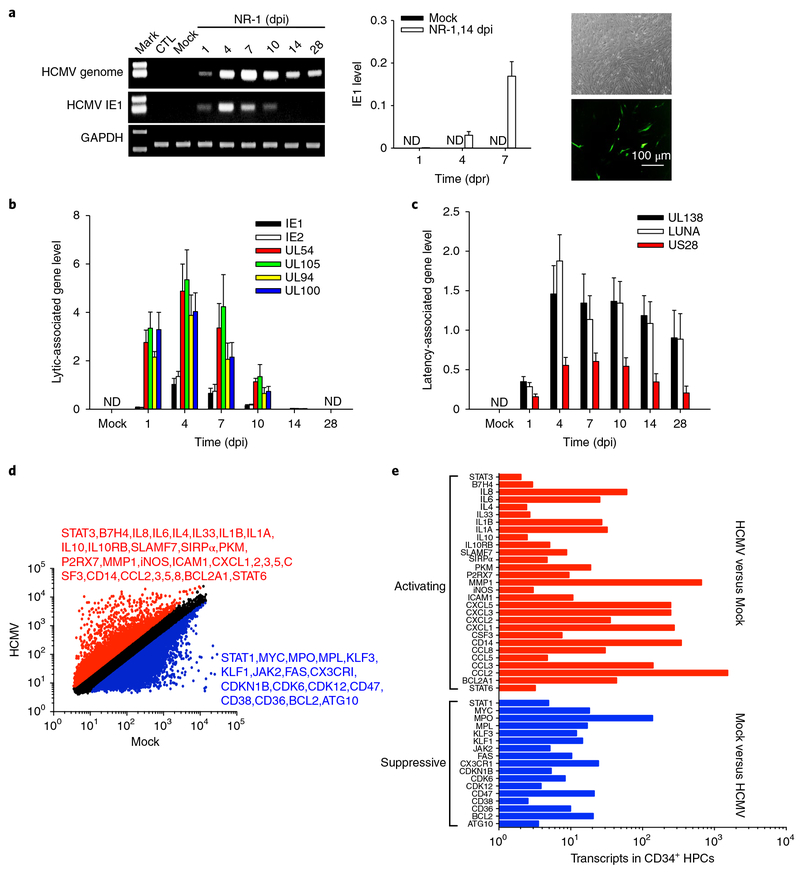
Fig. 1 |. HCMV NR-1 infection reprogrammes human CD34+ HPCs to achieve latent infection.
a, NR-1 successfully established latency in HPCs. HPCs isolated from bone marrow were infected with NR-1 or deactivated NR-1 (Mock) at a multiplicity of 2 p.f.u per cell. Virus was reactivated by TPA (20 ng ml−1) followed by coculture with HFF-1 cells. Left, levels of HCMV genome and IE1 in HPCs following NR-1 or Mock infection. Middle and right panels represent the quantitative PCR with reverse transcription result of IE1 expression and virus replication of GFP-expressing NR-1 after reactivating virus from latent infection in HPCs (NR-1, 14 dpi), respectively. b, Levels of HCMV IE1, IE2 and lytic infection-associated genes UL54, UL94, UL100 and UL105 in HPCs following the infection with NR-1 or Mock. c, Levels of HCMV latency-associated genes UL138, LUNA and US28 in HPCs following the infection with NR-1 or Mock. d, Alteration of transcription profiling of CD34+ HPCs by NR-1 latent infection (14 dpi). The red-coloured molecules are upregulated (fold change > 2) by NR-1 infection compared to Mock infection, whereas the blue-coloured molecules are downregulated (fold change < 0.5) by NR-1 infection compared to Mock infection. e, The activating (red-coloured) and suppressive (blue-coloured) signal pathways in NR-1 latently infected HPCs compared to those in Mock-infected HPCs. Data are presented as the mean ± s.e.m. of three independent experiments. CTL, control; ND, not detected; dpr, days post reactivation.