ABSTRACT
Background
The beverage hydration index (BHI) is a composite measure of fluid balance after consuming a test beverage relative to water. BHI is a relatively new measure that has been explored in young, but not yet older, adults.
Objective
The aim of this study was to investigate potential differences in BHI between euhydrated younger and older adults after drinking 4 different commercial beverages. We hypothesized that 1) older subjects would remain in positive fluid balance longer than young subjects after ingestion of each test beverage due to decreased urinary excretion rates, 2) glucose (glu)- and amino acid (AA)-based hydration beverages with sodium would have a BHI greater than water in both groups, and 3) the traditional 2-h postingestion BHI may be inappropriate for older adults.
Methods
On 5 separate visits, 12 young (23 ± 3 yr, 7 M/5F) and 12 older (67 ± 6 yr, 5 M/7F) subjects consumed 1 L of distilled water, G-20 (6% CHO, 20 mmol/L Na+), G-45 (2.5% CHO, 45 mmol/L Na+), AA-30 (5 AAs, 30 mmol/L Na+), or AA-60 (8 AAs, 60 mmol/L Na+) over 30 min. Blood and urine samples were collected before ingestion and at 0, 60, 120, 180, and 240 min postingestion with additional venous blood sampling at 5, 10, 15, and 30 min postingestion.
Results
In young subjects, BHI increased with increasing beverage Na+ concentration, and AA-60 had the highest BHI (AA-60 = 1.24 ± 0.10 compared with water = 1.00, P = 0.01). For older subjects, BHI was highest in AA-30 (AA-30; 1.20 ± 0.13 compared with water, P < 0.01) and was still in flux beyond 2 h in AA-60 (P < 0.05).
Conclusions
Beverage Na+ content progressively increased BHI in young adults independent of glucose or AA content. For older adults, the AA-30 beverage had the highest BHI. A 4-h BHI may be more appropriate for older adults due to attenuated urine excretion rates. This trial was registered at clinicaltrials.gov as NCT03559101.
Keywords: net fluid balance, healthy aging, eGFR, plasma volume, BHI
Introduction
As the number of Americans over the age of 65 yr increases, the need for research specific to the older population and its accompanying physiological alterations has intensified (1). Maintenance of euhydration and renal handling of ingested fluid (water and electrolytes) loads are challenges to homeostasis that may be altered in healthy aging (2–4). Adults over the age of 65 are more likely to become dehydrated and to sustain that dehydration longer than their younger counterparts due to attenuated thirst sensations and changes in renal conservation of fluid (5, 6). In health, most older adults maintain adequate hydration throughout the day, but often self-suppress fluid intake in the evenings and at night to avoid frequent urination (7). When illness strikes, older adults are often given an oral hydration solution to correct suspected dehydration and/or promote short-term hydration; yet little is known about which beverages might be most appropriate for this age cohort.
Traditional hydration products (oral rehydration solutions and sports drinks) containing glucose can readily restore hydration by promoting an increase in fluid absorption from the small intestine to the vascular space using Na+-dependent transporters (8). However, the carbohydrate content of these beverages may be undesirable in some circumstances. For example, glucose tolerance is known to decrease with age (9–11), and high glucose loads could be poorly tolerated with age-related glucose malabsorption (12). In addition to gut Na+-dependent glucose transporters, Na+-dependent amino acid (AA) gut transporters (13, 14) also facilitate water and electrolyte absorption comparably to glucose (15) and appear to maintain functionality in response to aging (16). As such, hydration solutions containing AAs in place of glucose may be similarly beneficial in maximizing fluid absorption and maintaining fluid balance in older adults without the additional calories (10) or potential to exacerbate challenges to glucose metabolism (9–11).
The beverage hydration index (BHI) is a relatively new quantitative index used to define the short-term hydration efficacy of a given beverage (17) and is standardized to the consumption of an equal volume of distilled water. In calculating BHI, 2-h urine output following ingestion of 1 L distilled water is standardized to a value of 1.0, and beverages with BHI > 1.0 exhibit attenuated urine output (greater fluid retention) compared to an equal volume of water, whereas beverages with BHI < 1.0 exhibit greater urine output (less fluid retention) than water. The 2-h value was chosen because any impact of fluid intake compared to water or among beverages was evident at 2 h and did not change over the subsequent 2 h in young adults (17).
Age-related differences in fluid handling may result from alterations in renal function, gastric emptying, and/or intestinal absorption (2–4). The potential for commercial optimization of oral rehydration solutions for older adults led us to study the differences in fluid balance and BHI after consumption of 1 L of 4 commercial carbohydrate (CHO)- or amino acid-based rehydration beverages in young (18–30 y) and older (60–85 y) adults. BHI has been previously studied only in young men and women (9, 10) and is independent of body size and sex (18). We hypothesized that 1) older subjects would remain in positive fluid balance longer than younger subjects after ingestion of each beverage due to decreased urinary excretion rates (2, 3), 2) glucose- and AA-based hydration beverages containing sodium would have a BHI higher than water in both groups (17, 18), and 3) the original 2-h postingestion BHI may be inappropriate for older adults because of slower urinary excretion rates.
Methods
Study population
Twenty-four subjects participated in this study: 12 young subjects between the ages of 18 and 30 yr and 12 older subjects aged 60 to 85 yr (Supplemental Figure 1), recruited from the community in Centre County, PA. Subjects were recruited using advertisements or from a pool of individuals who had participated in previous trials. All subjects underwent a screening exam including anthropometric measurements, resting heart rate and blood pressure, and blood chemistry (Chem-24 panel). Subjects were excluded if they had existing or suspected renal, cardiovascular, metabolic, or prostate diseases, or if they were taking any medications that could alter fluid balance. All procedures were approved by the Pennsylvania State University Institutional Review Board, and all subjects gave written and verbal consent before participation according to the Declaration of Helsinki. All testing was conducted at the Pennsylvania State University.
Study design
Subject characteristics are presented in Table 1. Twelve young and 12 older subjects participated in the study. All 12 young subjects completed all trials. Ten older subjects completed all trials, 1 older subject completed all trials except AA-60, and 1 older subject completed all trials except G-20 and G-45. Subject groups were matched for weight and BMI. The older group was significantly older by design and had a lower estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease formula (19).
TABLE 1.
Subject characteristics
| Young (7 M/5F) | Older (5 M/7F) | |
|---|---|---|
| Age, yr | 23 ± 3 | 68 ± 7* |
| Weight, kg | 73 ± 3 | 74 ± 4 |
| BMI, kg m–2 | 23 ± 1 | 26 ± 5 |
| Fasting glucose, mg dL–1 | 87 ± 1 | 100 ± 3* |
| Glycated hemoglobin, % | 5.1 ± 0.1 | 5.5 ± 0.1* |
| eGFR, mL min–1 1.73 m–2 | 94 ± 6 | 71 ± 3* |
| Urea nitrogen, mg dL–1 | 16 ± 1 | 19 ± 1 |
| Creatinine, mg dL–1 | 0.92 ± 0.07 | 0.92 ± 0.04 |
| BUN/creatinine ratio | 17.0 ± 1.1 | 19.9 ± 1.0 |
| Serum Na+, mmol L–1 | 137 ± 0.2 | 138 ± 0.2 |
| Urine Na+, mmol L–1 | 70 ± 7 | 64 ± 4 |
| S osm, mOsm kg–1 | 287 ± 1 | 293 ± 0 |
| U osm, mOsm kg–1 | 569 ± 36 | 447 ± 27 |
Data are means ± SEMs. BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; Na+, sodium concentration; Sosm, serum osmolality; Uosm, urine osmolality. *P < 0.05 compared to young.
The experimental procedures mimicked those described by Maughan et al. (17) in the original BHI development paper. Subjects refrained from vigorous exercise for 24 h, and caffeine and alcohol for 12 h before each experiment, and underwent an overnight fast for ≥8 h before each experiment. In addition, subjects were restricted from food intake during the experiment. The total period of food restriction did not produce any unintended consequences in any of the subjects. All subjects provided a first morning urine sample in a sterile collection container. One hour before arriving at the lab, subjects drank 500 mL of spring water (Aquafina, PepsiCo, Inc.) in a 15-min time period. Upon arrival at the laboratory, subjects voided their bladder into a 1-L plastic urine container (“pre” time point for urine) and near-nude body mass was measured. Subjects then sat for 10 min before an intravenous catheter was inserted into an antecubital vein. The preingestion blood sample (“pre” time point for blood) was collected. The subjects then began the 30-min drinking protocol, during which they drank 1 L of a randomly assigned test beverage in 4 equal aliquots (0.25 L every 7.5 min). Drink order was randomized by random number generation for each group, conducted by a research technician. Subjects were blinded to the commercial product names of the test beverages. After the subject drank the test beverage, blood samples were collected 0, 30, 60, 120, 180, and 240 min postingestion. Additional blood samples taken 5, 10, and 15 min postconsumption were subsequently added after the study began (water: young n = 6, older n = 7; G-20: young n = 3, older n = 6; G-45: young n = 5, older n = 6; AA-30: young n = 3, older n = 8; AA-60: young n = 4, older n = 4) to better capture early plasma volume (PV) changes. Urine samples were collected in 1-L urine containers after blood sampling at 0, 60, 120, 180, and 240 min postingestion. Blood samples were collected in 2 serum separator tubes (4 mL of blood each) to measure serum electrolytes and serum osmolality, and 1 K2 EDTA tube (3 mL) to measure hematocrit and hemoglobin.
Test beverages
Each participant completed the protocol 5 times, 1 for each test beverage, in a randomized study design (Supplemental Figure 1). Experimental trials were separated by a minimum of 1 wk to account for any residual effects of the test beverages. The test beverages were: distilled water, G-20, AA-30, G-45, and AA-60. Beverage composition is presented in Table 2 and was recorded from the product label with the exception of osmolality, which was measured in the laboratory. Beverage energy densities were 21, 105, and 237 kcal/L for AAs (30 and 60), G-45, and G-20, respectively. All drinks were kept sealed and stored at 4–6°C until serving.
TABLE 2.
Beverage composition
| Beverage | CHO (%) | Amino acids, number | Sodium, mmol/L | Potassium, mmol/L | Osmolality, mosm/kg |
|---|---|---|---|---|---|
| Water | 0 | 0 | 0 | 0 | 0 ± 0 |
| G-20 | 6 | 0 | 20 | 3.2 | 326 ± 20 |
| AA-30 | 0 | 5 | 30 | 10 | 145 ± 1 |
| G-45 | 2.5 | 0 | 45 | 20 | 320 ± 1 |
| AA-60 | 0 | 8 | 60 | 20 | 221 ± 1 |
Data are means ± SEMs. G-20, Gatorade; AA-30, enterade Advanced Oncology; G-45, Pedialyte; AA-60, enterade Anti-Diarrheal. Amino acids included in AA-60: aspartic acid, isoleucine, lysine, serine, threonine, tyrosine, valine, glycine. Amino acids included in AA-30: aspartic acid, serine, threonine, tyrosine, valine. CHO, carbohydrate.
Urine and blood analyses
Subjects voided completely into 1-L plastic urine containers at each time point. If subjects needed to urinate between scheduled collection times, urine was collected and combined with the urine collection of the following hour. Urine mass was weighed to the nearest ±0.1 g using an electronic balance, and urine osmolality was measured at each time point in triplicate using a freezing-point osmometer (Model 3320, Advanced Instruments, Inc.; percentage CV = 1.1%). Serum separator tubes remained upright for 30 min so the serum had the opportunity to clot before centrifuging (10 min, 4°C, 4000 rpm). The K2 EDTA tube and 1 serum separator tube from each time point were analyzed (Quest Diagnostics) for hematocrit, hemoglobin concentration, and serum electrolyte concentrations. One serum separator tube remained in the laboratory for serum osmolality analysis (Model 3320, Advanced Instruments, Inc.).
Calculations
Net fluid balance, BHI, change in PV, free water clearance (CH2O), and time in positive fluid balance were calculated. Net fluid balance was calculated by subtracting the cumulative urine output from the initial fluid load of 1000 g. BHI was calculated as the cumulative urine output of water (at 2 h or 4 h) divided by the cumulative urine output of the test beverage at the same time point. Change in PV was calculated using the method of Dill and Costill (20). The fractional reabsorption of solute and renal dilution capacity were estimated using CH2O (mL/min), calculated for each aggregate 2-h beverage BHI, where: CH2O = V̇ – Cosm, where V̇ was urine flow in mL/min; Cosm was osmolar clearance (mL/min) = Uosm × V̇ / Posm, where Uosm and Posm were urine and plasma osmolality (mOsm/kg), respectively. A plot of CH2O (y-axis) versus V̇ (x-axis) represents an index of urine dilution capacity (y = renal solute reabsorption, x = solute delivery) (21, 22) in response to the varying beverage solute loads delivered in identical 1-L volumes. Subjects were considered to be in positive fluid balance as long as the volume of fluid ingested minus the volume of urine excreted was >0. For the regression net fluid balance data were modeled using exponential nonlinear regression for each individual subject (model: y = (y0—Plateau)(–kx) + Plateau, GraphPad Prism 7), and the exact time at which net fluid balance was equal to zero was extrapolated for each subject from his or her individual regression.
Data and statistical analysis
Student's unpaired t tests were used to compare subject characteristics. Based on previous findings (17) and expected effect size of 1.09 for oral rehydration solution (G-45), we determined a priori (α = 0.05; power = 0.8) that 12 subjects per group would be sufficient to detect within- and between-group differences. Data were analyzed using PROC MIXED 2-way ANOVA (beverage × time) for within-group differences or 3-way ANOVA (beverage × time × group) for between-group differences (SAS 9.4). Statistical significance was set a priori and accepted at α < 0.05. When main effects were significant, Bonferroni post hoc analyses were performed to correct for multiple comparisons. Outliers outside 2 SDs from the mean were removed. Primary outcomes were BHI, net fluid balance, and changes in PV. Ordinary least-squares regression was used to examine the CH2O compared with V relation, and to compare slope and intercept terms between young and old. Data are presented as means ± SEM.
Results
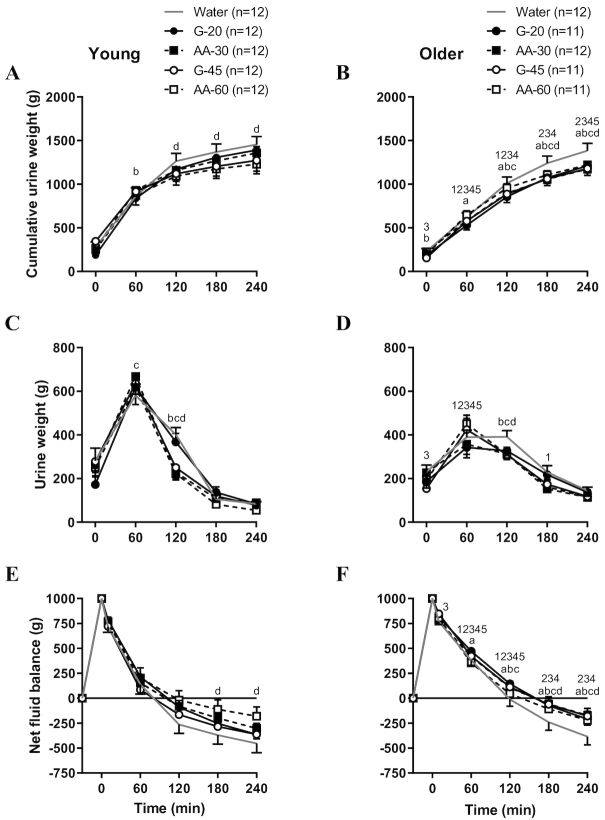
In the young subjects, the AA-60 beverage resulted in significantly less cumulative urine output at the end of the protocol than water (water: 1443.1 ± 97.0 g, AA-60: 1228.7 ± 108.9 g, P < 0.001; Figure 1A). For older adults, cumulative urine output after water consumption was significantly higher than all 4 Na+-containing hydration beverages (Figure 1B). Compared to young, older subjects produced significantly less total urine by 4 h following G-20, G-45, AA-30, and AA-60 (Figure 1A and B). Sixty minutes after beverage consumption, older adults produced less urine following all beverages than young adults (all P < 0.05; Figure 1C and D).
FIGURE 1.
Cumulative (A, B) and time point (C, D) urine output and fluid balance (E, F) in young and older adults. Values are means ± SEM. Differences within and between groups were assessed by 2-way and 3-way ANOVA, respectively, with Bonferroni post hoc analyses performed to correct for multiple comparisons. aP < 0.05 G-20 compared to water; bP < 0.05 G-45 compared to water; cP < 0.05 AA-30 compared to water; dP < 0.05 AA-60 compared to water; 1P < 0.05 water compared to young; 2P < 0.05 G-20 compared to young; 3P < 0.05 G-45 compared to young; 4P < 0.05 AA-30 compared to young; 5P < 0.05 AA-60 compared to young.
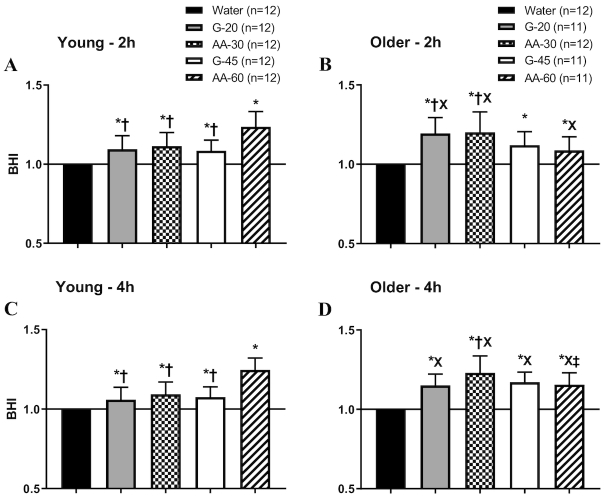
The BHI value for water was normalized to 1.0 for each individual subject in both groups and at both 2-h and 4-h calculated time points (Figure 2). BHI was significantly greater than 1.0 for all test beverages at both 2 and 4 h postconsumption for both age groups. The BHI did not change from 2 to 4 h in young subjects, but continued to increase from 2 h to 4 h in older subjects, significantly so in AA-60 (P < 0.05), due to delayed urinary excretion. In young subjects, the beverage with the highest sodium content (AA-60) produced a significantly higher BHI than all other beverages but was significantly lower in older adults than in young. G-20 and AA-30 produced significantly higher BHI than AA-60 after 2 h in older adults (Figure 2B). At 4 h, BHI for G-20, G-45, and AA-30 was significantly higher for older adults than for young (Figure 2C and D).
FIGURE 2.
Beverage hydration index (BHI) for the 4 test beverages in young and older adults at 2 h (A, B) and 4 h (C, D). Values are means ± SEM. Differences within and between groups were assessed by 2-way and 3-way ANOVA, respectively, with Bonferroni post hoc analyses performed to correct for multiple comparisons. *P < 0.05 compared to water; †P < 0.05 compared to AA-60; XP < 0.05 compared to young; ‡P < 0.05 compared to 2 h.
When viewed across time (Table 3), older adults spent more time in positive fluid balance than young adults. Young adults reached negative fluid balance with all ingested beverages between 1 and 2 h, whereas older subjects stayed in positive fluid balance until just before the 3-h point, with the exception of the water trial (Figure 1E and F). Figure 3 presents the estimated glomerular filtration rate (eGFR) versus time in positive fluid balance for the water trial. The lower the eGFR, the longer the time spent in positive fluid balance after consumption of 1 L of distilled water. There was a significant relation between eGFR and time spent in positive fluid balance for the older subjects (R2 = 0.47, P = 0.01), but not for young subjects (R2 = 0.10, P = 0.38). This fact helps explain BHI differences between young and old (2).
TABLE 3.
Time spent in positive fluid balance1
| Beverage | Young, min | Older, min |
|---|---|---|
| Water | 73.6 | 118.0 |
| G-20 | 94.7 | 156.5 |
| AA-30 | 88.4 | 147.0 |
| G-45 | 73.2 | 152.2 |
| AA-60 | 103.9 | 124.7 |
1Time spent in positive fluid balance using the model: y = (y0 – Plateau)(–kx) + Plateau. Data are means. G-20, Gatorade; AA-30, enterade Advanced Oncology; G-45, Pedialyte; AA-60, enterade Anti-Diarrheal.
FIGURE 3.
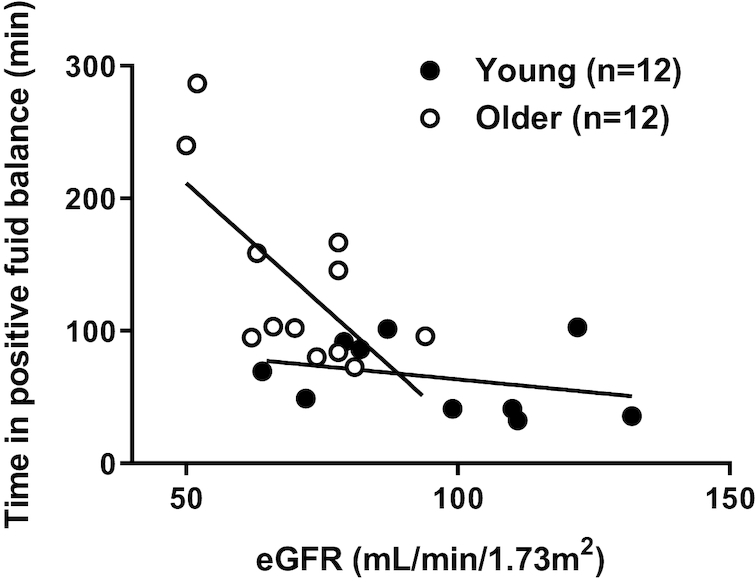
Relation between estimated glomerular filtration rate (eGFR) and time spent in positive fluid balance in young compared to older adults. Separate ordinary least-squares regression analyses assessed the correlation between eGFR and time in positive fluid balance in young and older adults. Older: R2 = 0.47,P = 0.01; young: R2 = 0.10, P = 0.38.
Figure 4 compares CH2O production as an index of beverage solute reabsorption, across varying rates of renal filtrate delivery (4 commercial beverages). CH2O was a linear function of V̇ below and parallel to the line of identity. There were no differences in the slope (P = 0.40) or y-intercept (P = 0.34) terms between young and old. The proportionality between CH2O and V̇ was constant despite differences in eGFR, and remained unchanged when adjusted for eGFR (data not shown). The equation: CH2O = 0.829 × V̇ – 1.81, formed the best-fit line for both groups, whereby CH2O contributed 50–70% of the total V̇ across beverages tested. This suggests that renal handling of solute was similar between young and old, despite age-related reductions in CH2O and delayed solute excretion sometimes reported (2, 3).
FIGURE 4.
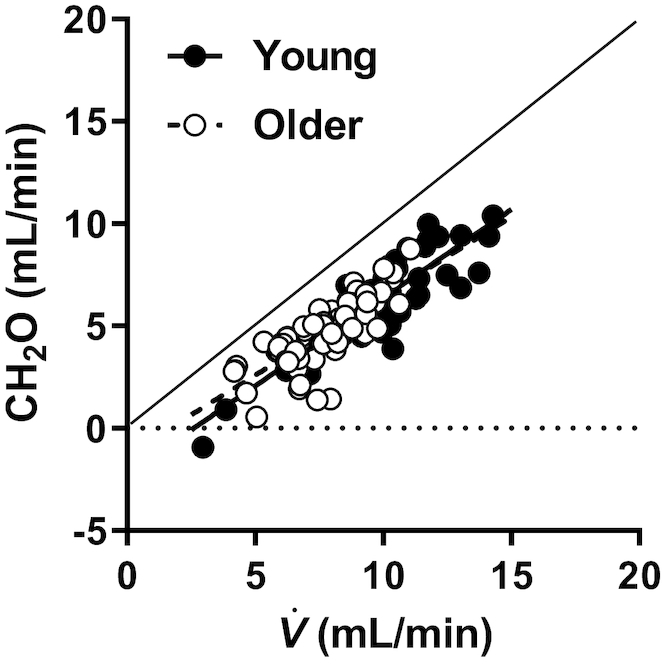
Comparison of free water clearance (CH2O; index of solute reabsorption) at varying urine flows (V̇; index of solute delivery) between old and young subjects. Ordinary least-squares regression analyses assessed the correlation between CH2O and V̇ in young and older adults. Each subject is represented a total of 5 times, once for each test beverage, at the 2-h time point. Older: R2 = 0.59, P < 0.01; young: R2 = 0.76, P < 0.01. Neither the slope (P = 0.40) nor the intercept (P = 0.34) was different between groups.
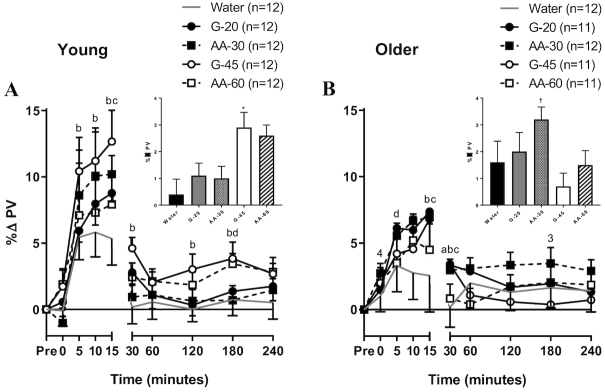
Figure 5 presents changes in PV within the first 15 min postingestion (line graphs) and followed for the subsequent 4 h (line graphs and composite bar graphs). Older adults had an attenuated initial peak in PV compared to young subjects (P < 0.05). In both young and older adults, the initial rise in PV was greater following G-45 and AA-30 ingestions than following water (Figure 5). In young adults, PV remained elevated for the final 3 h after ingestion of the highest 2 Na+-containing beverages, significantly so after G-45 consumption (P < 0.05), compared to water (Figure 5A inset). Older adults had the highest sustained PV expansion after consumption of AA-30 (P < 0.05; Figure 5B inset). After ingestion of G-45, older adults had significantly lower sustained PVs than after ingestion of all other beverages and significantly lower sustained PVs than young adults.
FIGURE 5.
Plasma volume changes (% baseline) across the 4 h protocol in young (A) and older (B) adults. Values are means ± SEM. Differences within and between groups were assessed by 2-way and 3-way ANOVA, respectively, with Bonferroni post hoc analyses performed to correct for multiple comparisons. Additional plasma volume measurements were made in a subgroup of subjects at 5-, 10-, and 15-min time points, with the following sample sizes. Water: young n = 6, older n = 7; G-20: young n = 3, older n = 6; G-45: young n = 5, older n = 6; AA-30: young n = 3, older n = 8; AA-60: young n = 4, older n = 4. aP < 0.05 G-20 compared to water; bP < 0.05 G-45 compared to water; cP < 0.05 AA-30 compared to water; dP < 0.05 AA-60 compared to water; 3P < 0.05 G-45 compared to young; 4P < 0.05 AA-30 compared to young. The inset bar graph is the mean of the 60 min to 240 min %PV change for each beverage to represent the plateau phase. *P < 0.05 compared to water. †P < 0.05 compared to water, G-20, G-45, and AA-60.
Discussion
The present investigation extends the use of the newly developed BHI to healthy older adults. The primary findings in our young subject cohort confirmed that, after ingestion of a 1-L bolus of fluid, the BHI for young adults generally increased with graded increases in Na+ content, regardless of whether the drink was CHO- or AA-based. We extended those findings to demonstrate that increased Na+ content of beverages allowed for prolonged PV expansion. In the older subjects, the time spent in positive fluid balance increased due to decreases in eGFR. Differences across age and beverage were not stable at the traditional 2-h time point for BHI calculation but changed between 2 and 4 h in the older group, in whom net fluid balance was prolonged with all Na+-containing drinks, but the drinks were undifferentiated (Figure 1F). At 4 h postingestion, the AA-30 beverage had the highest BHI.
BHI is a composite score that combines gastric emptying, intestinal absorption, and renal handling of beverages that differ in composition. Previous examinations of BHI in young subjects have shown that BHI is not altered by BMI or sex (18), but neither examined age or whether the retained fluid stayed in the vascular space. As expected, all of the Na+-containing test beverages had BHI > 1.0, which indicated that more of the ingested fluid was retained inside the body relative to water. The highest BHI was associated with the highest beverage Na+ content (AA-60) in young adults. At the level of the kidney, free water clearance is proportional to urine flow rate (Figure 4) and inversely proportional to beverage Na+ concentrations (data not shown). Thus, BHI differences between beverages may be due to differences in absorption and excretion patterns, but only a tracer technique would allow differentiation (23). Similarly, BHI is a useful noninvasive comparative technique to determine hydration efficacy (i.e., how much ingested fluid is retained in the body), but does not determine where the fluid is located within the body.
Older adults may benefit from beverages that maintain a positive fluid balance for a longer period of time (than with water) to hydrate more rapidly and to avoid inconveniences in urination (24, 25). Although CHO-based beverages have traditionally been used for generic hydration occasions, a liter of sports drink provides 80% of the total carbohydrate load (60 g) (Table 2) and ∼50% of the glycemic load (26) administered in a glucose tolerance test beverage (typically 75 g of glucose), which may not be ideal for aging populations prone to disorders of glucose metabolism (9–11, 27). As such, there is a need for alternative hydration options specifically for older adults, which may be served by AA-Na+ beverages (Figure 2).
BHI within age groups is normalized for differences in eGFR (i.e., normalized to eGFR behavior in response to water). Although differences in eGFR explain the longer time in positive hydration in older subjects (Figure 3) (2) and the possible need for a longer (4 h) test (Figure 2), neither eGFR nor renal solute handling (Figure 4) clearly explains differences in BHI between groups or within groups, among beverages. This finding may be due to the smaller group differences in eGFR than in other studies (2, 3) or less absolute impairment in filtration (21). Between-group differences beyond eGFR are more speculative (28), but the simplest explanation for the differences within groups could be that the commercial beverages tested differed in electrolyte composition, osmolality, and/or the ability to activate intestinal substrate transporters—one or all of which may have affected gastric emptying, intestinal absorption, and beverage retention (29).
In our study design, changes in PV immediately following fluid ingestion (e.g., the rapid rise in the first ∼15 min) likely reflect the rapid absorption of the ingested fluid (30), followed by a fall (∼15–30 min) and establishment of a prolonged plateau across the remaining 3.5 h of the protocol. Compared to young, older adults had an attenuated initial increase in PV in the first 15 min. However, both older and young adults maintained an equal expansion (∼3%) in the plateau from 1 h to 4 h. The attenuated initial peak may indicate a slower absorption of the beverages in the small intestine in older adults. This may be beneficial for older adults, because it may mitigate large changes in blood pressure and/or reduce the need to urinate immediately after ingestion, both of which may not be as important for young adults (31). In the current study, all test beverages caused a larger initial rise in PV than in the water trial, and the greatest initial increase in PV in the young subjects followed G-45 consumption. The 2 test beverages with the greatest Na+ content maintained a higher PV across the protocol than water alone in the young subject group. This finding is likely explained by the high plasma Na+ retaining water within the vascular space (32–34). These PV changes indicate that fluid remained in the vascular space and was not in the interstitial space, intestine, or bladder. Because the entire 1 L volume of fluid was excreted in the presence of a modest PV expansion, the hypotonic nature of the urine (Figure 4, 50–70% CH2O) suggests that electrolytes were retained and/or higher extracellular tonicity created a favorable osmotic pull of water from the intracellular space (35). The PV response in concert with BHI adds value, because mild expansion of the vascular space is a beneficial outcome related to fluid therapy (36).
Clinical implications and future directions
A previous study developing the BHI described the 2-h BHI for a number of different beverages in healthy young adults (17). That study demonstrated no discriminating difference among beverages in BHI values beyond 2 h, concluding that 2 h was sufficient to determine the BHI, and the 2-h BHI calculation was suggested as a standardized methodology. However, our data demonstrate that older adults had differences in BHI after 2 h, although only statistically significant in AA-60, explained by a lower eGFR that was significantly related to the rate of urine output (i.e., time to excrete 1 L of water) in this study. Owing to the prolonged urine excretion profile in older adults, our data suggest that caution should be used in reporting 2-h BHI values for older adults, and that it may be more appropriate to report a 4-h BHI when comparing beverages in the older cohort. Furthermore, differences in BHI between young and old at 2 h could not be explained by eGFR or renal solute handling.
Our data suggested that AA-based hydration products work as well as, and possibly better than, CHO-based products for maintaining positive fluid balance in older adults. The drinks tested in the current study are commercially available (except AA-60) and are used or marketed to correct dehydration, which is a common condition in older adults. Nocturia is also common in this population and may compound dehydration via fluid avoidance. The BHI of beverages marketed to correct dehydration, particularly those that do not depend on the sodium-coupled glucose transporter (i.e., AA-based) are worthy of study to assess and compare their retention profiles for these reasons. Our measures focused on urinary excretion and changes in vascular volume. However, we did not directly examine potential effects on gastric emptying and intestinal absorption. As such, future research should investigate gastric emptying and intestinal absorption after ingestion of these beverages to better characterize the overall fate of ingested fluids.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Susan Slimak, RN, and Jane Pierzga, MS, for assistance.
The authors’ responsibilities were as follows—WLK, SNC, and RWK: designed research; MMC and STW: conducted research; MMC, AES, SNC, RWK, and WLK: analyzed data; MMC, STW, AES, SNC, RWK, and WLK: wrote and edited manuscript; WLK: had primary responsibility for final content; and all authors: read and approved the final manuscript. None of the authors report a conflict of interest related to research presented in this article.
Notes
Partially funded by an unrestricted gift from Entrinsic Health Solutions, Inc. and by NIH T-32 Grant #5T32AG049676-03.
The opinions or assertions contained herein are the private views of the authors and should not be construed as official or reflecting the views of the Army or the Department of Defense. Any citations of commercial products, organizations, and trade names in this report do not constitute an official Department of the Army endorsement of approval of the products or services of these organizations. Approved for public release: distribution unlimited.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AA, amino acid; AA-30, enterade Advanced Oncology; AA-60, enterade Anti-Diarrheal; BHI, beverage hydration index; CH2O, free water clearance; eGFR, estimated glomerular filtration rate; G-20, Gatorade; G-45, Pedialyte; PV, plasma volume.
References
- 1. Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation. 2003;107(1):139–46. [DOI] [PubMed] [Google Scholar]
- 2. Crowe MJ, Forsling ML, Rolls BJ, Phillips PA, Ledingham JG, Smith RF. Altered water excretion in healthy elderly men. Age Ageing. 1987;16(5):285–93. [DOI] [PubMed] [Google Scholar]
- 3. Fish LC, Murphy DJ, Elahi D, Minaker KL. Renal sodium excretion in normal aging: Decreased excretion rates lead to delayed handling of sodium loads. Geriatr Nephrol Urol. 1994;4(3):145–51. [Google Scholar]
- 4. Mack GW, Weseman CA, Langhans GW, Scherzer H, Gillen CM, Nadel ER. Body fluid balance in dehydrated healthy older men: Thirst and renal osmoregulation. J Appl Physiol. 1994;76(4):1615–23. [DOI] [PubMed] [Google Scholar]
- 5. de Castro JM. Age-related changes in natural spontaneous fluid ingestion and thirst in humans. J Gerontol. 1992;47(5):P321–30. [DOI] [PubMed] [Google Scholar]
- 6. Rowe JW, Shock NW, DeFronzo RA. The influence of age on the renal response to water deprivation in man. Nephron. 1976;17(4):270–8. [DOI] [PubMed] [Google Scholar]
- 7. Kenney WL, Chiu P. Influence of age on thirst and fluid intake. Med Sci Sports Exerc. 2001;33(9):1524–32. [DOI] [PubMed] [Google Scholar]
- 8. Gisolfi CV, Lambert GP, Summers RW. Intestinal fluid absorption during exercise: Role of sport drink osmolality and [Na+]. Med Sci Sports Exerc. 2001;33(6):907–15. [DOI] [PubMed] [Google Scholar]
- 9. Stolk RP, Pols HA, Lamberts SW, de Jong PT, Hofman A, Grobbee DE. Diabetes mellitus, impaired glucose tolerance, and hyperinsulinemia in an elderly population. The Rotterdam Study. Am J Epidemiol. 1997;145(1):24–32. [DOI] [PubMed] [Google Scholar]
- 10. Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD et al.. Mechanisms of the age-associated deterioration in glucose tolerance: Contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52(7):1738–48. [DOI] [PubMed] [Google Scholar]
- 11. De Tata V. Age-related impairment of pancreatic Beta-cell function: Pathophysiological and cellular mechanisms. Front Endocrinol. 2014;5(138). doi:10.3389/fendo.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drozdowski L, Woudstra T, Wild G, Clandindin MT, Thomson AB. The age-associated decline in the intestinal uptake of glucose is not accompanied by changes in the mRNA or protein abundance of SGLT1. Mech Ageing Dev. 2003;124(10-12):1035–45. [DOI] [PubMed] [Google Scholar]
- 13. Curran PF, Schultz SG, Chez RA, Fuisz RE. Kinetic relations of the Na–amino acid interaction at the mucosal border of intestine. J Gen Physiol. 1967;50(5):1261–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang EB, Sitrin MD, Black DD. Gastrointestinal, hepatobiliary, and nutritional physiology. Philadelphia: J.B. Lippincott Company. [Google Scholar]
- 15. Tai CY, Joy JM, Falcone PH, Carson LR, Mosman MM, Straight JL, Oury SL, Mendez C Jr., Loveridge NJ, Kim MP et al.. An amino acid-electrolyte beverage may increase cellular rehydration relative to carbohydrate-electrolyte and flavored water beverages. Nutr J. 2014;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferraris RP, Vinnakota RR. Regulation of intestinal nutrient transport is impaired in aged mice. J Nutr. 1993;123(3):502–11. [DOI] [PubMed] [Google Scholar]
- 17. Maughan RJ, Watson P, Cordery PA, Walsh NP, Oliver SJ, Dolci A, Rodriguez-Sanchez N, Galloway SD. A randomized trial to assess the potential of different beverages to affect hydration status: Development of a beverage hydration index. Am J Clin Nutr. 2016;103(3):717–23. [DOI] [PubMed] [Google Scholar]
- 18. Sollanek KJ, Tsurumoto M, Vidyasagar S, Kenefick RW, Cheuvront SN. Neither body mass nor sex influences beverage hydration index outcomes during randomized trial when comparing 3 commercial beverages. Am J Clin Nutr. 2018;107(4):544–9. [DOI] [PubMed] [Google Scholar]
- 19. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T et al.. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37(2):247–8. [DOI] [PubMed] [Google Scholar]
- 21. Kahn T, Mohammad G, Stein RM. Alterations in renal tubular sodium and water reabsorption in chronic renal disease in man. Kidney Int. 1972;2(3):164–74. [DOI] [PubMed] [Google Scholar]
- 22. Martínez-Maldonado M, Opava-Stitzer SC. Pathophysiology of clinical disorders of urine concentration and dilution In: Pathophysiology of the Kidney. Office of the Dean (RGP). 1977;5:992–1028. [Google Scholar]
- 23. Hill RJ, Bluck LJC, Davies PSW. The hydration ability of three commercially available sports drinks and water. J Sci Med Sport. 2008;11(2):116–23. [DOI] [PubMed] [Google Scholar]
- 24. Johnson TM 2nd, Sands JM, Ouslander JG. A prospective evaluation of the glomerular filtration rate in older adults with frequent nighttime urination. J Urol. 2002;167(1):146–50. [PubMed] [Google Scholar]
- 25. Willis-Gray M, Wu JM, Markland A. Urinary incontinence and hydration: A population-based analysis. Neurourol Urodyn. 2018;37(1):200–5. [DOI] [PubMed] [Google Scholar]
- 26. Shi X, Summers RW, Schedl HP, Flanagan SW, Chang R, Gisolfi CV. Effects of carbohydrate type and concentration and solution osmolality on water absorption. Med Sci Sports Exerc. 1995;27(12):1607–15. [PubMed] [Google Scholar]
- 27. Poulsen SB, Fenton RA, Rieg T. Sodium–glucose cotransport. Curr Opin Nephrol Hypertens. 2015;24(5):463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sands JM. Urine concentrating and diluting ability during aging. J Gerontol Ser A. 2012;67(12):1352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leiper JB. Fate of ingested fluids: Factors affecting gastric emptying and intestinal absorption of beverages in humans. Nutr Rev. 2015;73(suppl_2):57–72. [DOI] [PubMed] [Google Scholar]
- 30. Endo Y, Torii R, Yamazaki F, Sagawa S, Yamauchi K, Tsutsui Y, Morikawa T, Shiraki K. Water drinking causes a biphasic change in blood composition in humans. Pflugers Arch. 2001;442(3):362–8. [DOI] [PubMed] [Google Scholar]
- 31. Frey MA, Riddle J, Charles JB, Bungo MW. Blood and urine responses to ingesting fluids of various salt and glucose concentrations. J Clin Pharmacol. 1991;31(10):880–7. [DOI] [PubMed] [Google Scholar]
- 32. Kamijo Y, Ikegawa S, Okada Y, Masuki S, Okazaki K, Uchida K, Sakurai M, Nose H. Enhanced renalNa+reabsorption by carbohydrate in beverages during restitution from thermal and exercise-induced dehydration in men. Am J Physiol Regul Integr Comp Physiol. 2012;303(8):R824–33. [DOI] [PubMed] [Google Scholar]
- 33. Shi X, Summers RW, Schedl HP, Chang RT, Lambert GP, Gisolfi CV. Effects of solution osmolality on absorption of select fluid replacement solutions in human duodenojejunum. J Appl Physiol. 1994;77(3):1178–84. [DOI] [PubMed] [Google Scholar]
- 34. Musso CG, Alvarez Gregori J, Jauregui JR, Macias Nunez JF. Creatinine, urea, uric acid, water and electrolytes renal handling in the healthy oldest old. World J Nephrol. 2012;1(5):123–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nose H, Mack GW, Shi XR, Nadel ER. Shift in body fluid compartments after dehydration in humans. J Appl Physiol. 1988;65(1):318–24. [DOI] [PubMed] [Google Scholar]
- 36. Cheuvront SN, Kenefick RW, Charkoudian N, Mitchell KM, Luippold AJ, Bradbury KE, Vidyasagar S. Efficacy of glucose or amino acid-based commercial beverages in meeting oral rehydration therapy goals after acute hypertonic and isotonic dehydration. JPEN J Parenter Enteral Nutr. 2018;42(7):1185–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.