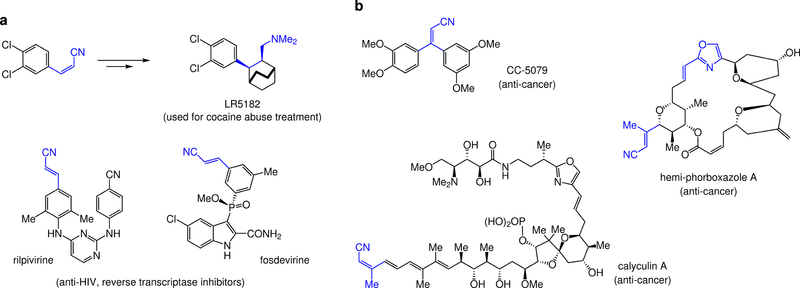
Figure 1. Biologically active compounds with an alkenyl nitrile or a related moiety.
a, Stereoisomerically pure 1,2-disubstituted olefins bearing a nitrile substituent may be used to prepare medicinally relevant agents, such as LR5182, a polycyclic tertiary amine used to battle cocaine abuse. Furthermore, stereochemically defined alkenyl nitriles reside in a range of biologically active molecules. Examples are rilpivirine and fosdevirine, entities relevant to the fight against AIDS. b, Stereoisomerically pure trisubstituted alkenyl nitriles are desirable as well. These moieties are found in biologically active entities, represented by anti-cancer agents CC-5079, various phorboxazoles, and calyculin A. In the case of phorboxazoles, the oxazole ring and its adjacent olefin may be generated from an alkenyl nitrile as well.