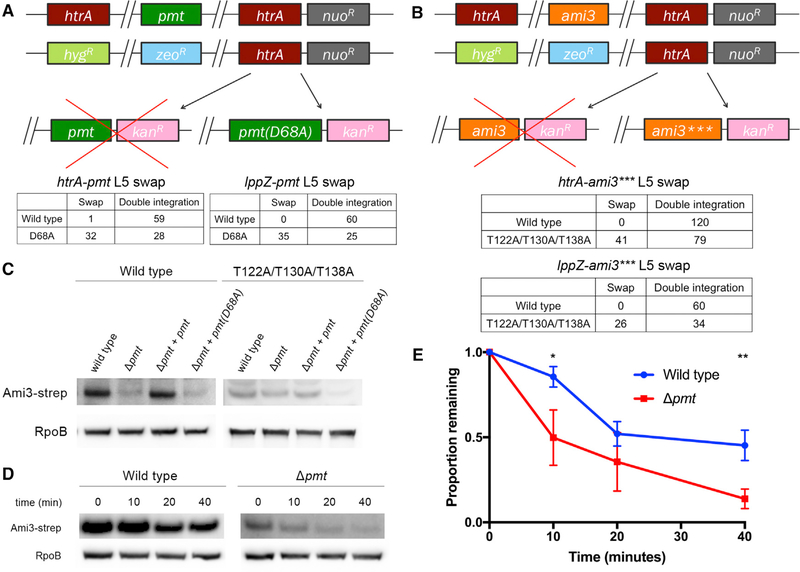
Figure 5. Pmt Mannosylation Stabilizes Ami3.
(A) Mutating the catalytic activity of Pmt suppresses the essentiality of htrA and IppZ. Top: the endogenous copies of pmt and htrA were replaced with zeocin and hygromycin resistance cassettes, respectively, and a copy of htrA was integrated at the L5 phage integration site. pmt or pmt(D68A), a catalytically inactive variant of pmt, was transformed into this background. Full swaps that acquire kanamycin resistance at the expense of nourseothricin resistance render strains devoid of htrA (or IppZ) and must thus carry a suppressor mutation. Bottom: quantification of pmt and pmt(D68A) swaps. A total of 60 transformants were tested for antibiotic resistance. An equivalent swap was performed for IppZ and enumerated in the same manner.
(B) Removing the mannosylation residues of Ami3 suppresses the essentiality of htrA and IppZ. Top: the endogenous copies of ami3 and htrA were replaced with zeocin and hygromycin resistance cassettes, respectively, and a copy of htrA was integrated at the L5 phage integration site. ami3 or ami3(T122A/T130A/T138A) (ami3***), an allele of ami3 in which all mannosylation sites have been mutated (see Figure S4), was transformed into this background. Full swaps that acquire kanamycin resistance at the expense of nourseothricin resistance render strains devoid of htrA (or lppZ) and must thus carry a suppressor mutation. Bottom: quantification of ami3 and ami3*** swaps. A total of 120 transformants were tested for antibiotic resistance. An equivalent swap was performed for lppZ and enumerated in the same manner; a total of 60 transformants were tested for antibiotic resistance.
(C) Ami3 levels decrease in cells missing Pmt. Strains expressing Ami3-Strep or Ami3*** in a wild-type, Δpmt, Δpmt+pmt, or Δpmt+pmt(D68A) background were grown to log phase. Whole-cell lysate was analyzed using western blotting using anti-Strep and anti-RpoB as a loading control.
(D) Pmt mannosylation increases the stability of Ami3 protein. At indicated time points after adding chloramphenicol, aliquots of cells were lysed, and levels of Ami3-Strep were monitored using western blot. Results are representative of three independent experiments.
(E) Quantification of Ami3 stability. Independent values of RpoB-normalized levels of Ami3 from (D) quantified using densitometry analysis. Western blot images were cropped but display all relevant lanes and reactive bands. *p < 0.05 and **p < 0.01. Error bars represent SD of the mean.