Figure 2.
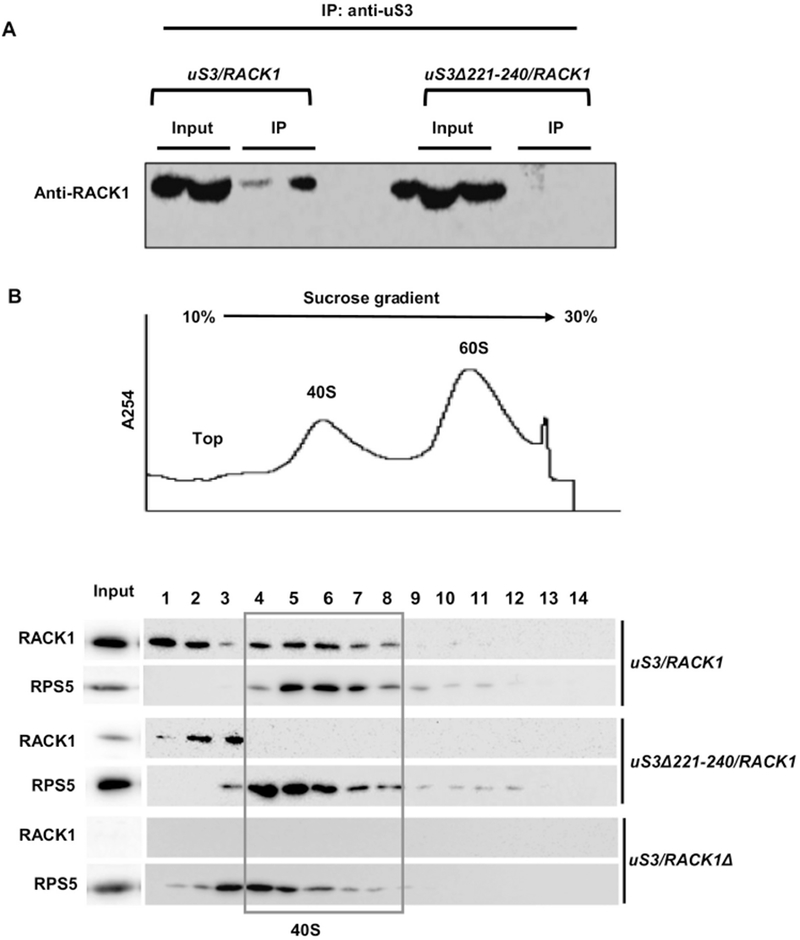
uS3-RACK1 interaction is essential for ribosomal recruitment of RACK1: (A) Co-immunoprecipitation analysis showing that deletion of the last 20 aa residues from uS3-CTD abrogates RACK1 interaction with the ribosome; (B) Polysome profiling analysis showing that RACK1 is unable to associate with the mutant 40S ribosomal subunits harboring uS3Δ221-240. Whole cell extracts from wild-type and mutant strains were resolved in 10-30% sucrose gradients and the fractions were probed to detect the presence of RACK1 with the anti-RACK1 antibody. A representative UV trace at 254 nm is shown above. RACK1 does not bind to the 40S in the uS3Δ221-240/RACK1 strain. Input - represents a 7% portion of each gradient fraction. Western blotting with anti-rpS5 antibody is also shown to detect the 40S subunit fractions.
