Table 1.
Decarboxylative Trifluoromethylation: Initial Studiesa
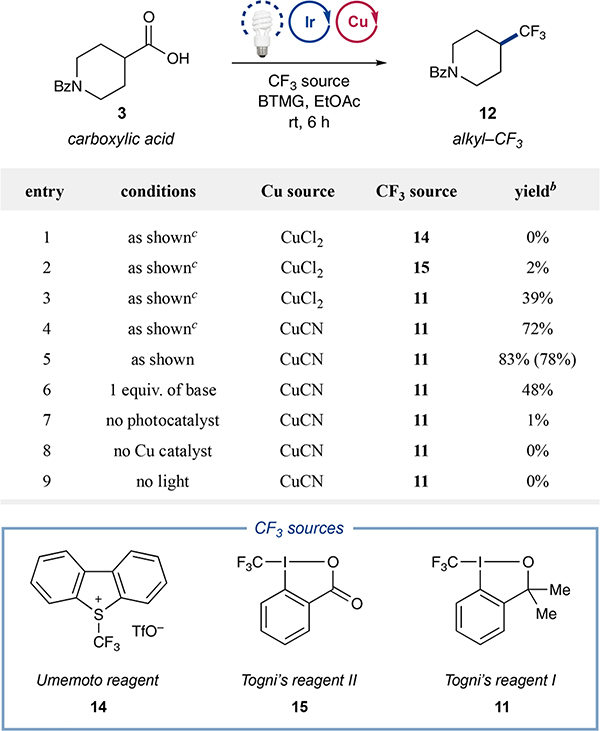 |
Performed with acid 3 (0.05 mmol), photocatalyst 1 (1 mol%), Cu source (20 mol%), bathophenanthroline (30 mol%), 2-tert-butyl-1,1,3,3-tetramethylguanidine (BTMG, 0.5 equiv), CF3 source (1.25 equiv), and H2O (30 equiv) in EtOAc (0.025 M).
Yields by 19F NMR analysis. Yields in parentheses are isolated yields.
The ligand 3,4,7,8-tetramethyl-1,10-phenanthroline was used on the Cu catalyst.
