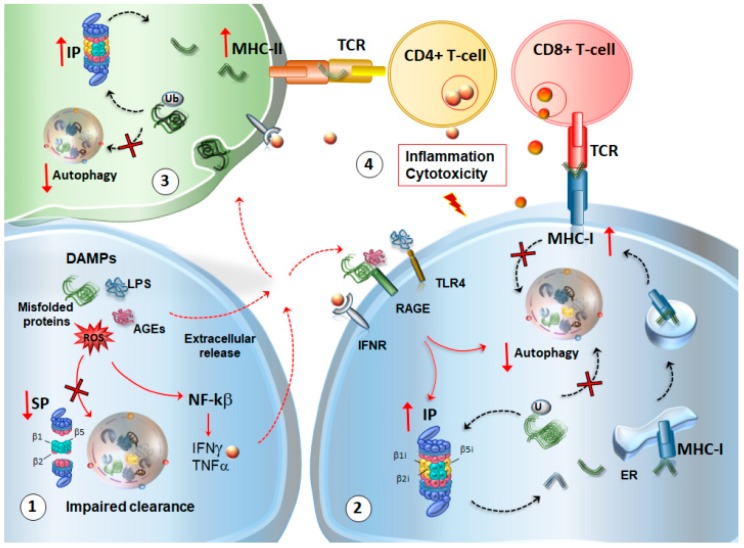
Figure 4.
Molecular mechanisms bridging neuro-inflammation, immunoproteasome (IP) induction and autophagy-SP dysfunction in the central nervous system (CNS). (1) As a consequence of autophagy and SP dysregulation, indigested misfolded or oxidized substrates may perpetuate inflammation through the release of danger-associated molecular pattern molecules (DAMPs), such as advanced glycation end-products (AGEs), lipopolysaccharides (LPS), and ROS. While DAMPs activate NF-κB and the inflammasome to release cytokines such as interferon gamma (IFNγ), the spreading of indigested misfolded proteins, AGEs, and free radicals in the extracellular space occurs. (2) All of these factors converge to induce an upregulation of IP within the neighboring cells via autocrine or paracrine mechanisms. DAMPs may also stimulate AGE receptors, IFN receptors, and toll-like 4 receptor (TLR4), to converge on molecular pathways such as mTOR, which, in turn, induce IP upregulation and SP-autophagy downregulation. In this scenario, IP upregulation leads to an overproduction of CNS self-antigens co-expressed with major histocompatibility complex (MHC)-I molecules to prime cytotoxic CD8+ T cell response. At the same time, autophagy cannot efficiently provide for the internalization of MHC-I molecules or the degradation of damaged proteins and organelles. (3) Within glial cells, the same DAMPs and cytokines that foster glial activation may contribute to the impairment of autophagy and SP, while up-regulating IP, which is able to process and cross-present phagocytosed proteins via MHC-II for the activation of CD4+ T cells. (4) In this way, IP upregulation, in an attempt to compensate for SP-autophagy downregulation, may fuel inflammation and auto-immune activation to promote altered synaptic plasticity and neuronal damage. Red arrows in bold indicate decreased/increased levels/activity of SP, IP, autophagy, MHC-I, MHC-II. Plain red arrows indicate intra-cellular signaling cascades. Dotted red arrows indicate the extracellular release of DAMPs, cytokines, and their binding to cognate receptors in neighbor cells or phagocytosis by glial cells. Dotted black arrows indicate the shuttling of substrates towards UPS or autophagy, the formation of Ag peptides deriving from UPS cleavage, and the progression of MHC-I-Ag complex from the ER and endosomes to the plasma membrane. The red frame indicates the final effect produced by CD4+ and CD8+ T-cells activation on neurons and glia.