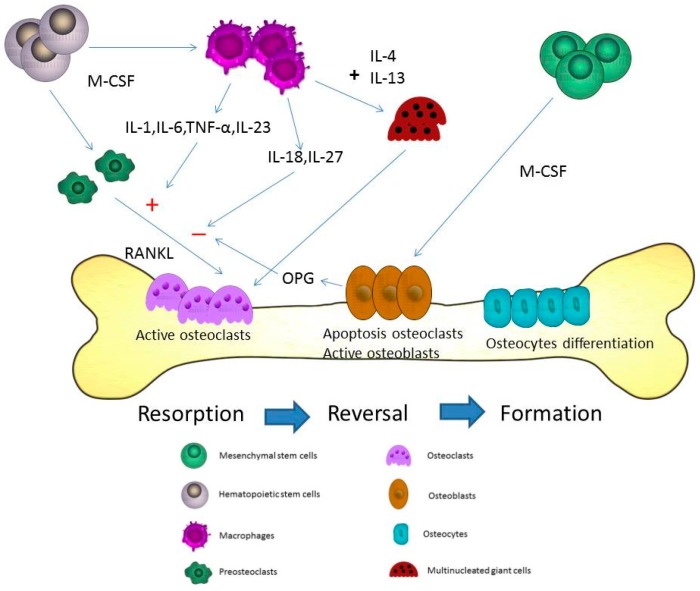
Figure 1.
Microdamages in the bone-remodeling units of cancellous or cortical bone induced by osteoclasts. Osteoclasts may be activated by different cytokines including receptor activator of NF-κB ligand (RANKL), interleukin (IL)-1, IL-6, and macrophage-colony stimulating factor (M-CSF) in the resorption state. After the resorption process, the reversal state progresses. In the reversal state, apoptosis of osteoclasts may be induced to stop the bone resorption. The replacement of osteoblasts is observed at the same time. The activated osteoblasts refill the resorption pits and tunnels on the bone surface. In the formation state, osteoblasts directly adhere to the bone surface and progressively form into osteocytes. The proliferation of osteocytes can use these lacuna-canalicular networks to connect within the bone matrix. The cytokines from macrophages including IL-6, tumor necrosis factor-α (TNF-α), IL-23, IL-18, and IL-27 can induce and inhibit osteoclastogenesis through RANKL in bone remodeling. M-CSF is the most important cytokine in the initial stage of macrophage differentiation from hematopoietic stem cells. Different phases are observed during the formation of the multinucleation. RANKL is the major cytokine that stimulates osteoclasts into mature multinucleated osteoclasts. The cytokines, IL-4 and IL-13, may induce macrophages to form multinucleated giant cells during the course of bone resorption.