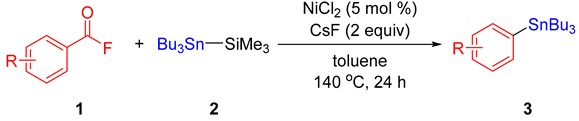
Table 2.
Decarbonylative stannylation of acyl fluorides a,b.
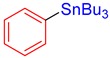 3a, 90% |
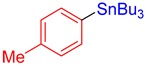 3b, 81% |
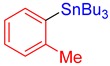 3c, 63% |
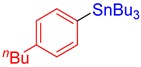 3d, 67% |
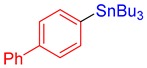 3e, 82% |
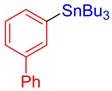 3f, 56% |
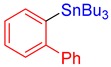 3g, 85% |
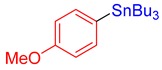 3h, 64% |
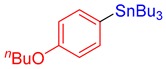 3i, 61% |
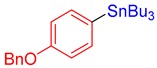 3j, 50% |
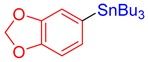 3k, 87% |
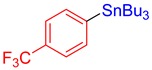 3l, 61% |
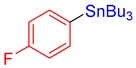 3m, 86% |
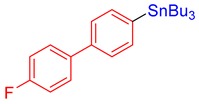 3n, 72% |
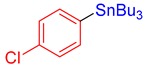 3o, 90% |
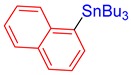 3p, 51% |
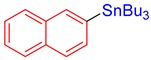 3q, 79% |
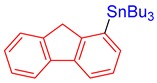 3r, 82% |
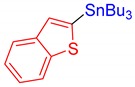 3s, 62% |
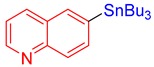 3t, 84% |
aReaction conditions: 1 (0.2 mmol), 2 (0.24 mmol), NiCl2 (0.01 mmol), CsF (0.4 mmol), toluene (1 mL), 140 °C, 24 h. b Isolated yields.
