Abstract
A reduction in BCR-ABL1/ABL1IS transcript levels to <10% after 3 months or <1% after 6 months of tyrosine kinase inhibitor therapy are associated with superior clinical outcomes in chronic myeloid leukemia (CML) patients. In this study, we investigated the reliability of multiple BCR-ABL1 thresholds in predicting treatment outcomes for 184 subjects diagnosed with CML and treated with standard-dose imatinib mesylate (IM). With a median follow-up of 61 months, patients with concordant BCR-ABL1/ABL1IS transcripts below the defined thresholds (10% at 3 months and 1% at 6 months) displayed significantly superior rates of event-free survival (86.1% vs. 26.6%) and deep molecular response (≥ MR4; 71.5% vs. 16.1%) compared to individuals with BCR-ABL1/ABL1IS levels above these defined thresholds. We then analyzed the outcomes of subjects displaying discordant molecular transcripts at 3- and 6-month time points. Among these patients, those with BCR-ABL1/ABL1IS values >10% at 3 months but <1% at 6 months fared significantly better than individuals with BCR-ABL1/ABL1IS <10% at 3 months but >1% at 6 months (event-free survival 68.2% vs. 32.7%; p < 0.001). Likewise, subjects with BCR-ABL1/ABL1IS at 3 months >10% but <1% at 6 months showed a higher cumulative incidence of MR4 compared to patients with BCR-ABL1/ABL1IS <10% at 3 months but >1% at 6 months (75% vs. 18.2%; p < 0.001). Finally, lower BCR-ABL1/GUSIS transcripts at diagnosis were associated with BCR-ABL1/ABL1IS values <1% at 6 months (p < 0.001). Our data suggest that when assessing early molecular responses to therapy, the 6-month BCR-ABL1/ABL1IS level displays a superior prognostic value compared to the 3-month measurement in patients with discordant oncogenic transcripts at these two pivotal time points.
Keywords: chronic myeloid leukemia, BCR-ABL1, imatinib mesylate, European Leukemia Net, early molecular response
1. Introduction
Chronic myeloid leukemia (CML) is characterized by a unique cytogenetic marker, the Philadelphia (Ph) chromosome, arising from the reciprocal translocation between the long arms of chromosomes 9 and 22 [1]. In turn, the Ph chromosome generates the BCR-ABL1 fusion chimeric gene, encoding an oncoprotein with constitutive tyrosine kinase activity that alters the proliferation rate, survival signaling, immunological interactions, and cytoskeletal dynamics of the hematopoietic stem cell [2,3,4,5,6,7]. The introduction of the tyrosine kinase inhibitor (TKI) imatinib mesylate (IM) has radically improved the outcome of chronic phase CML patients by generating unprecedented rates of complete hematological (CHR) and cytogenetic (CCyR) responses and deep molecular responses (MR) [8,9]. Despite these excellent results, IM resistance is often detected in patients failing to achieve an optimal response (OR) as defined by the current European Leukemia Net (ELN) recommendations [10]. IM resistance includes both BCR-ABL1-dependent [11,12,13] and BCR-ABL1-independent mechanisms [14,15] that may be prevented or overcome by second- (2G) or third-generation (3G) TKIs such as dasatinib (DAS), nilotinib (NIL), bosutinib (BOS) and ponatinib (PON). Moreover, non-ABL1-directed inhibitors and immunological-targeting approaches are currently being developed as additional treatment strategies for the disease [16,17,18].
With the introduction of 2G and 3G TKIs, the early identification of CML patients at high risk of failing IM treatment has become of pivotal importance. Hence, several clinical prognostic scores [Sokal and EUTOS long-term survival (ELTS)] have been employed to predict CML response to IM at the time of diagnosis, thereby recognizing patients that will display inferior overall survival (OS) rates [19,20,21,22]. At the same time, several groups have begun to investigate early molecular parameters that might distinguish CML patients unlikely to benefit from IM. A seminal paper by Marin et al. reported that BCR-ABL1/ABL1 transcript thresholds of 10% at 3 months and 1% at 6 months strongly predict long-term outcomes for CML patients [23]. Subsequently, Hanfstein and colleagues reported that BCR-ABL1/ABL1IS levels >1% at 6 months were associated with inferior 5-year OS compared to values <1%, thereby suggesting that BCR-ABL1 transcripts at 6 months predict the response of CML to IM [24]. This body of evidence has been gradually incorporated into clinical practice. Currently, both the National Comprehensive Cancer Network (NCCN) and ELN guidelines include the failure to achieve selected molecular responses, albeit at different time points, as a reason to switch to a different TKI.
In this complex clinical and molecular scenario, a challenging issue is how to manage patients who display discordant BCR-ABL1/ABL1IS transcripts (BCR-ABL1/ABL1IS <10% at 3 months but >1% at 6 months or BCR-ABL1/ABL1IS >10% at 3 months but <1% at 6 months) at the 3- and 6-month time points. In this study, we investigated the clinical implications of these molecular landmarks, in subjects with discordant transcripts at the 3- and 6-month time points, in order to translate these molecular data into clinically meaningful information for CML patients receiving IM as first-line treatment for their disease.
2. Results
2.1. Patient Responses and ELN Outcomes
Patient characteristics are summarized in Table 1. Every patient achieved a CHR, 157 (85.3%) attained a CCyR, 143 (77.7%) reached a major molecular response (MR3) (median time 10 months; range, 3–83), and 90 (48.9%) achieved a deep molecular response (MR4) (median time 20.5 months; range, 6–83). Median follow-up of the accrued population was 61 months (range, 12–90). According to the 2013 ELN recommendations, 126 (68.5%) patients achieved an optimal response, 39 (21.2%) failed IM, 10 (5.4%) were classified as “warning”, and 9 (4.9%) discontinued IM due to drug intolerance. All individuals who discontinued IM because of drug failure or intolerance received 2G TKIs.
Table 1.
Patient characteristics (n = 184).
| Characteristics | % | |
|---|---|---|
| Follow-Up (Median mo.) | 61 | |
| Age (Years) | ||
| Median | 55 | |
| Range | 20–87 | |
| Sex (pts n.) | ||
| Male | 94 | 51.1 |
| Female | 90 | 48.9 |
| Sokal Risk Group (pts n.) | ||
| Low/Int | 146 | 79.3 |
| High | 38 | 20.7 |
| ELTS Risk Group (pts n.) | ||
| Low/Int | 163 | 88.6 |
| High | 21 | 11.4 |
| Transcript Type | ||
| e13a2 (b2a2) | 74 | 40.2 |
| e14a2 (b3a2) | 92 | 50 |
| e13a2 and e14a2 | 18 | 9.8 |
| Optimal Response (pts n.) | 126 | 68.5 |
| Warning (pts n.) | 10 | 5.4 |
| Intolerant (pts n.) | 9 | 4.9 |
| Failure (pts n.) | 39 | 21.2 |
ELTS: EUTOS long-term survival.
2.2. Probability of Event-Free Survival and Molecular Response According to BCR-ABL1/ABL1IS Transcripts at 3 and 6 Months
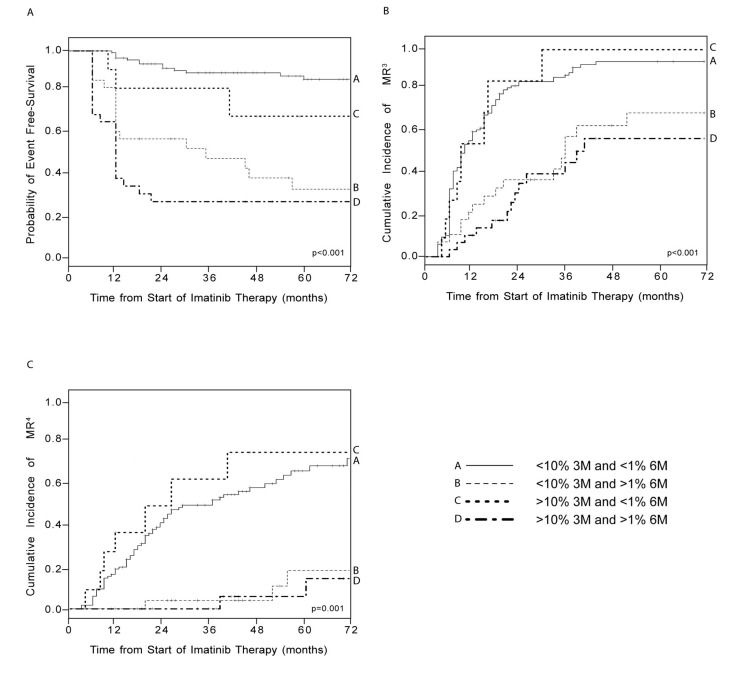
Consolidated data has established that BCR-ABL1/ABL1IS levels of 10% at 3 months and 1% at 6 months are predictive of OS, progression-free survival (PFS), event-free survival (EFS), and CCyR [23,24]. When we stratified patients according to their BCR-ABL1/ABL1IS levels at 3 and 6 months, we found that 63% of the patients had concordant low transcripts (i.e., <10% at 3 months and <1% at 6 months, group A) while 15.8% of individuals displayed concordant high transcripts (i.e., >10% at 3 months and >1% at months, group D). EFS probability was 86.1% vs. 26.6% (p < 0.001) in patients respectively displaying concordant low values (group A) or concordant high values (group D) (Figure 1A).
Figure 1.
Event-free survival (EFS) estimates (A) and cumulative incidence of major molecular response (MR3) (B) and deep molecular response (MR4) (C) according to BCR-ABL1/ABL1IS transcripts at 3 and 6 months. Patients were divided into four groups according to their BCR-ABL1 expression at the 3 and 6 months. EFS probability (A) and cumulative incidence of MR3 (B) and MR4 (C) were calculated for each group. Vertical lines indicate censored patients. p-values refer to statistical significance among all four groups included in the analyses.
The remaining 21.2% of patients displayed discordant transcript values at the 3- and 6-month time points, as 15.2% presented low (<10%) BCR-ABL1/ABL1IS at 3 months but high (>1%) levels at 6 months (group B), and 6% displayed high (>10%) BCR-ABL1/ABL1IS at 3 months, but low (<1%) levels at 6 months (group C). Among subjects with discordant BCR-ABL1 expression, group B (<10% at 3 months but >1% at 6 months) achieved significantly lower 6-year EFS as compared to group C (>10% at 3 months but <1% at 6 months) (32.7% vs. 68.2%, p < 0.001; Figure 1A).
We next evaluated if the 3- or the 6-month BCR-ABL1/ABL1IS levels would predict subsequent molecular responses to IM. Therefore, we compared the cumulative incidence of MR3 and MR4 in the four groups described above. As expected, there were significant differences between groups A and D. Specifically, patients with low transcripts at both 3 and 6 months showed significantly higher cumulative incidences of both MR3 (94.2% for group A vs. 57.1% for group D, p < 0.001; Figure 1B) and MR4 (71.5% for group A vs. 16.1% for group D, p < 0.001; Figure 1C). As for patients with discordant BCR-ABL1/ABL1IS levels, individuals in group B displayed significantly lower cumulative incidences of both MR3 (69.4% vs. 100% for group C, p < 0.001; Figure 1B) and MR4 (18.2% vs. 75% for group C, p < 0.001; Figure 1C).
2.3. Correlation between BCR-ABL1/GUSIS Levels at Diagnosis and BCR-ABL1/ABL1IS Transcripts at 3 and 6 Months
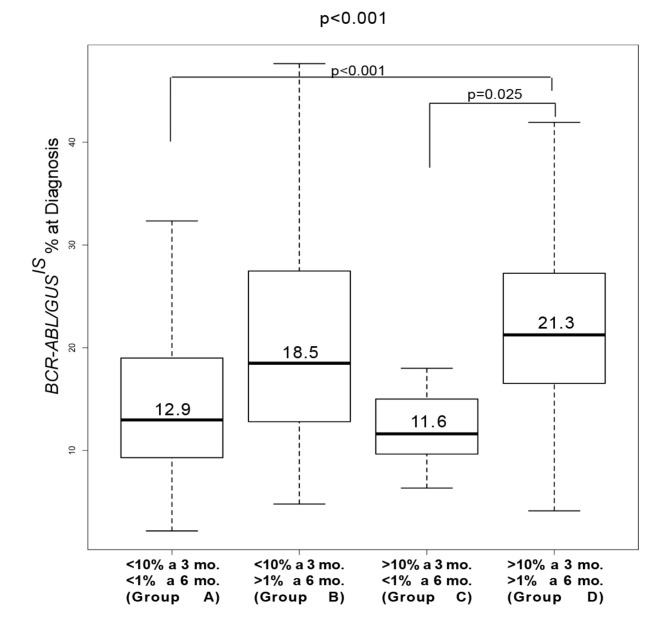
We and Bonecker have demonstrated that quantification of BCR-ABL1 transcripts at diagnosis by using GUS rather than ABL1 as a reference gene predicts IM response, as this measurement reflects the amount of BCR-ABL1 transcripts within each leukemic cell [25,26]. Therefore, we explored the correlation between BCR-ABL1/GUSIS values at diagnosis and BCR-ABL1/ABL1IS levels at 3 and 6 months. We found the highest BCR-ABL1/GUSIS transcripts at diagnosis in groups B (18.5%) and D (21.3%), which were those that presented BCR-ABL1/ABL1IS values >1% at 6 months. On the contrary, both groups A and C - displaying BCR-ABL1/ABL1IS values <1% at 6 months - expressed lower BCR-ABL1/GUSIS at diagnosis (12.9% for group A and 11.6% for group C). These differences were statistically significant (p < 0.001; Figure 2).
Figure 2.
Comparison between BCR-ABL1/GUSIS levels at diagnosis and BCR-ABL1/ABL1IS transcripts at 3 and 6 months. BCR-ABL1/GUSIS levels were determined for each group and depicted as boxplots delimited by the 25th (lower) and 75th (upper) percentile. Horizontal lines above and below each boxplot indicate the 5th and 95th percentile, respectively. Thick lines in each boxplot represent median BCR-ABL1/GUSIS in each patients group. The p-value above the figure refers to statistical significance among all four groups included in the analysis while p-values displayed inside the figure refer to statistical significance among the population groups indicated by the bracket (comparison between groups A and D: p < 0.001; comparison between groups C and D: p = 0.025).
2.4. Correlation between Risk Scores at Diagnosis and BCR-ABL1/ABL1IS Transcripts at 3 and 6 Months
Multiple findings have established that low Sokal and ELTS scores are associated with better OS [10,21,22]. We calculated that in our patient cohort, the Sokal and ELTS risk categories correlated with high or low BCR-ABL1/ABL1IS transcripts at 3 and 6 months. Patients were subdivided into two categories according to their Sokal or ELTS scores (to perform a binomial analysis, low and intermediate risk were grouped together and compared to high risk) and were then stratified into the four previously described groups (A–D) according to their BCR-ABL1/ABL1IS levels at 3 and 6 months. By considering the Sokal score, we found a significant correlation in groups with concordant BCR-ABL1/ABL1IS transcripts at 3 and 6 months (p < 0.02782; Table 2). Specifically, the group with the best outcome (A) presented a significantly higher percentage of low-/intermediate-risk patients as compared to the group (D) with the worst outcomes (p < 0.004). In groups with discordant transcripts, we found that individuals with BCR-ABL1/ABL1IS levels >1% at 6 months (group B) displayed a three-fold greater number of subjects with a high Sokal risk as compared to individuals with BCR-ABL1/ABL1IS levels >10% at 3 months (group C; 15.8% vs. 5.3%). However, these differences were not statistically significant. When we repeated this analysis considering the ELTS score, we again found a significant correlation between different risk groups and patients displaying concordant BCR-ABL1/ABL1IS transcripts at 3 and 6 months (p < 0.00592; Table 2). In details, more patients with low BCR-ABL1 expression at both time points (group A) presented low/intermediate ELTS scores compared to subjects with higher BCR-ABL1 levels at 3 and 6 months (group D; p = 0.0055). No significant correlation was achieved in groups with discordant BCR-ABL1 transcripts at the two time points, possibly due to the low number of patients in each of these patient subsets.
Table 2.
Association between risk scores and BCR-ABL1/ABL1IS at 3 and 6 months.
| BCR-ABL1/ABL1IS | Sokal Risk | p | |
|---|---|---|---|
| Low/Intermediate Risk (%) | High Risk (%) | ||
| n = 146 | n = 38 | ||
|
<10% @ 3 mo.
<1% @ 6 mo. |
98 (67.1) | 18 (47.3) | 0.02782 |
|
<10% @ 3 mo.
>1% @ 6 mo. |
22 (15) | 6 (15.8) | |
|
>10% @ 3 mo.
<1% @ 6 mo. |
9 (6.1) | 2 (5.3) | |
|
>10% @ 3 mo.
>1% @ 6 mo. |
17 (13.8) | 12 (31.6) | |
| BCR-ABL1/ABL1IS | ELTS Risk | p | |
| Low/Intermediate Risk | High Risk | ||
| n = 163 | n = 21 | ||
|
<10% @ 3 mo.
<1% @ 6 mo. |
105 (64.4) | 11 (52.4) | 0.00592 |
|
<10% @ 3 mo.
>1% @ 6 mo. |
27 (16.6) | 1 (4.8) | |
|
>10% @ 3 mo.
<1% @ 6 mo. |
11 (6.8) | 0 (0) | |
|
>10% @ 3 mo.
>1% @ 6 mo. |
20 (12.2) | 9 (42.9) | |
3. Discussion
Quantification of BCR-ABL1/ABL1IS transcripts after 3 and 6 months of TKI therapy has become routine practice for the management of CML, as BCR-ABL1 levels below the conventional 10% (at 3 months) and 1% (at 6 months) thresholds are associated with higher probabilities of achieving excellent failure-free survival (FFS), EFS, PFS, and OS [23,24,27]. As expected, in this report, we confirmed that patients displaying BCR-ABL1/ABL1IS transcripts below the indicated thresholds at both the 3- and 6-month landmarks presented superior outcomes compared to those with BCR-ABL1 transcripts above the 10% and 1% values. However, we also wanted to investigate the clinical impact of BCR-ABL1/ABL1IS transcripts when the values measured at the 3- and 6-month time points were discordant: i.e., a satisfactory (<10%) value followed by an unsatisfactory one (>1%) or vice versa. A previous publication by the Hammersmith group, authored by Neelakantan and colleagues, showed that when the 3 and 6 months transcripts were discordant, the 3-month levels displayed a superior prognostic value compared to the 6-month measurement [28]. Interestingly, we found an opposite result in our patient cohort, since in subjects with discordant 3 and 6-month transcripts, the latter were predicted to be significantly more responsive to treatment compared to the former. This discrepancy might be explained by differences in the number of low (39.9% vs. 28.9%) and high (20.7% vs. 28.9%) Sokal risk patients included in our study compared to the Hammersmith paper. Indeed, a population with a less aggressive disease, as described by a low Sokal score, would probably includes individuals with oncogenic transcripts >10% at 3 months that will continue to exhibit declining transcripts over time, therefore achieving additional benefit from further treatment with the same TKI. By contrast, a population with a more aggressive disease (high Sokal score) will probably include individuals displaying BCR-ABL1/ABL1IS transcripts >10% at 3 months that have already acquired resistance to IM and are unlikely to benefit from additional treatment with the same drug. This can justify the lower predictive value of BCR-ABL1 transcripts at the 6-month time point in the UK cohort. This hypothesis is in line with the different number of patients included in the group with high BCR-ABL1 transcripts at 3 months but low levels at 6 months in our series (6%), compared to the UK cohort (2%). It should also be noted that our results are in agreement with those previously published by the Korean group, indicating that BCR-ABL1 transcripts below the 1% threshold at 6 months are a reliable molecular parameter capable of identifying a subset of patients that will benefit from their assigned treatment even if their BCR-ABL1 levels at 3 months were above the 10% value [29]. Unfortunately, we could make no comparisons between the data summarized in the UK and Korean studies and our reported findings concerning the ELTS score, as this parameter only became available in 2016 and was therefore not included in previously published manuscripts.
Both the NCCN and the ELN recommendations for the management of CML patients require disease molecular evaluations at 3, 6, and 12 months during TKI treatments. In our previous study, we demonstrated that baseline quantification of BCR-ABL1 expression is a useful parameter to discriminate, at diagnosis, patients unlikely to benefit from standard-dose IM [25]. In the current report, we wanted to validate these data by correlating baseline BCR-ABL1 quantification with the molecular transcripts detected at 3 and 6 months. We confirm a direct association between BCR-ABL1 expression levels at diagnosis and IM response, as patients with BCR-ABL1 values <1% at 6 months (groups A and C) were those expressing lower BCR-ABL1 transcripts at diagnosis.
Finally, several clinical trials have demonstrated that patients who achieve and maintain a deep molecular response (≥MR4) could be considered for TKI discontinuation as they may remain in treatment-free remission (TFR), even after drug cessation. TFR is an attractive possibility because of relief from TKI toxicities, desire to plan a pregnancy, and general improvement in quality of life. Moreover, even with the advent of generic IM, TKI discontinuation may greatly relieve the financial toxicity associated with CML treatment [30]. Our data suggest that molecular responses at 3 and 6 months after IM may predict which patients will achieve greater benefit from the drug and should, therefore, be considered for treatment-free remission.
4. Materials and Methods
4.1. Patient Characteristics and Treatment
Between 1 June 2010, and 31 December 2017, a total of 184 adult patients with chronic phase (CP) CML were accrued to this study. Diagnosis of CP-CML was defined by conventional criteria. No prior treatment for CML other than hydroxyurea was allowed.
All patients received IM 400 mg daily as first-line therapy. The drug was discontinued in the presence of grade 3/4 toxicities with treatment resumed after toxicity reduction to grade 1 or complete resolution. IM responses were evaluated according to the 2013 ELN criteria [10]. Only those patients who had BCR-ABL1 transcripts at 3 and 6 months were included in this study.
The research ethics committee (Supplementary Materials) of each recruiting institution reviewed and approved the study protocol on 10 October 2005 and all patients gave written informed consent for the data to be used in this analysis.
4.2. Hematologic, Cytogenetic, and Molecular Analyses Response
A complete hematological response was defined as previously reported [31]. Cytogenetic analysis was performed at diagnosis, at 3 and 6 months, and then every 6 months until a CCyR was achieved. At least 20 bone marrow cell metaphases were evaluated through conventional G-banding analysis. CCyR was defined as the failure to detect any Philadelphia chromosome (Ph)-positive metaphases in two consecutive examinations [32]. Confirmed detection of one or more Ph-positive metaphases after acquiring a CCyR was considered a cytogenetic relapse.
BCR-ABL1 and ABL1 expression were measured from peripheral blood (PB) samples drawn at diagnosis and then every three months using real-time PCR (qPCR) as previously described [25]. ABL1 was used as the reference gene at any time point other than diagnosis. In addition, at diagnosis, BCR-ABL1 expression was measured by using GUS as a housekeeping gene as it is a more appropriate reference gene for specimens expressing high BCR-ABL1 [33]. All samples were processed for nucleic acid extraction in the Center of Experimental Oncology and Hematology of the A.O.U. Policlinico-Vittorio Emanuele, as previously described [34]. qPCR determinations for BCR-ABL1/ABL1 and BCR-ABL1/GUS were converted to the international scale (IS), as previously reported [25]. MR3 was defined by BCR-ABL1/ABL1IS ≤0.1% (3-log reduction from the standardized baseline) [35,36], while MR4 was defined by BCR-ABL1/ABL1IS ≤0.01% (≥4-log reduction from standardized baseline) [35,36]. qPCR determinations were considered of appropriate quality only in the presence of no less than 24,000 GUS copies or 10,000 ABL1 copies, as previously reported [36].
4.3. Statistical Analysis
Probabilities of event-free and failure-free survival, and cumulative incidence (CI) of different molecular responses, were calculated using the Kaplan–Meier method. Statistical significance between different Kaplan–Meier curves was evaluated using the Mantel–Haenszel test, as previously described [37]. Differences in BCR-ABL1/GUSIS, at diagnosis, in the four groups of patients defined according to their BCR-ABL1/ABL1IS levels at 3 and 6 months, were calculated using the ANOVA test, followed by the Tukey HSD post hoc test. Events included in our definition of EFS were death from any cause, a progression from chronic phase, IM failure according to the 2013 ELN recommendations, and development of intolerance. FFS was defined as survival without evidence of drug failure according to the latest ELN recommendations.
Differences in the occurrence of specific risk scores (Sokal or ELTS) among the four groups of patients were assessed using the Fisher exact test computing two-sided p-values with 95% confidence intervals. All statistical analyses were performed using R software [38].
Abbreviations
| ABL | Abelson murine leukemia |
| BOS | bosutinib |
| CCyR | complete cytogenetic response |
| CML | chronic myeloid leukemia |
| CHR | complete hematological response |
| DAS | dasatinib |
| ELN | European Leukemia Net |
| ELTS | EUTOS long-term survival |
| EFSFFS | event-free survivalfailure-free survival |
| GUS | β-glucuronidase |
| IM | imatinib mesylate |
| MR | molecular responses |
| NCCN | National Comprehensive Cancer Network |
| NIL | nilotinib |
| OR | optimal response |
| OS | overall survival |
| Ph | Philadelphia chromosome |
| PON | ponatinib |
| PFS | Progression-free survival |
| TKI | tyrosine kinase inhibitor |
| TFR | treatment-free remission |
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/20/9/2226/s1.
Author Contributions
F.S. designed the research, supervised the study, analyzed the data, interpreted the results, recruited and followed the patients, and wrote the paper; S.S. (Stefania Stella) designed the research, performed the experiments, analyzed the data, interpreted the results, and wrote the paper; P.V. designed the research, supervised the study, analyzed the data, interpreted the results, and wrote the paper; S.R.V., M.S.P., M.M. (Michele Massimino) and E.T. performed the experiments and critically reviewed the paper; S.F. performed statistical analysis and critically reviewed the paper; A.S. critically reviewed the paper; V.Z., A.A., S.S. (Sergio Siragusa), V.A., D.M., S.I., C.M., S.R., A.M., G.M., M.M. (Maurizio Musso), F.P. and B.M. recruited and followed the patients and critically reviewed the paper; L.M. interpreted the results and critically reviewed the paper; F.D.R. supervised the study, interpreted the results, and critically reviewed the paper.
Funding
This research received no external funding.
Conflicts of Interest
Fabio Stagno: honoraria from BMS, Incyte, Novartis, Pfizer. Francesco Di Raimondo: research funding from BMS, honoraria from Novartis, Incyte, BMS, and Pfizer. Paolo Vigneri: research funding from Novartis, honoraria from Astra-Zeneca, Celgene, Incyte, Novartis, and Pfizer. The other authors declare no conflict of interest.
References
- 1.Stagno F., Stella S., Spitaleri A., Pennisi M.S., Di Raimondo F., Vigneri P. Imatinib mesylate in chronic myeloid leukemia: Frontline treatment and long-term outcomes. Expert Rev. Anticancer Ther. 2016;16:273–278. doi: 10.1586/14737140.2016.1151356. [DOI] [PubMed] [Google Scholar]
- 2.Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat. Rev. Cancer. 2005;5:172–183. doi: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- 3.Massimino M., Consoli M.L., Mesuraca M., Stagno F., Tirro E., Stella S., Pennisi M.S., Romano C., Buffa P., Bond H.M., et al. IRF5 is a target of BCR-ABL kinase activity and reduces CML cell proliferation. Carcinogenesis. 2014;35:1132–1143. doi: 10.1093/carcin/bgu013. [DOI] [PubMed] [Google Scholar]
- 4.Giallongo C., Tibullo D., La Cava P., Branca A., Parrinello N., Spina P., Stagno F., Conticello C., Chiarenza A., Vigneri P., et al. BRIT1/MCPH1 expression in chronic myeloid leukemia and its regulation of the G2/M checkpoint. Acta Haematol. 2011;126:205–210. doi: 10.1159/000329911. [DOI] [PubMed] [Google Scholar]
- 5.Preyer M., Vigneri P., Wang J.Y. Interplay between kinase domain autophosphorylation and F-actin binding domain in regulating imatinib sensitivity and nuclear import of BCR-ABL. PLoS ONE. 2011;6:e17020. doi: 10.1371/journal.pone.0017020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stella S., Tirro E., Conte E., Stagno F., Di Raimondo F., Manzella L., Vigneri P. Suppression of survivin induced by a BCR-ABL/JAK2/STAT3 pathway sensitizes imatinib-resistant CML cells to different cytotoxic drugs. Mol. Cancer Ther. 2013;12:1085–1098. doi: 10.1158/1535-7163.MCT-12-0550. [DOI] [PubMed] [Google Scholar]
- 7.Manzella L., Tirro E., Pennisi M.S., Massimino M., Stella S., Romano C., Vitale S.R., Vigneri P. Roles of Interferon Regulatory Factors in Chronic Myeloid Leukemia. Curr. Cancer Drug Targets. 2016;16:594–605. doi: 10.2174/1568009616666160105105857. [DOI] [PubMed] [Google Scholar]
- 8.Druker B.J., Guilhot F., O’Brien S.G., Gathmann I., Kantarjian H., Gattermann N., Deininger M.W., Silver R.T., Goldman J.M., Stone R.M., et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 9.Hochhaus A., Larson R.A., Guilhot F., Radich J.P., Branford S., Hughes T.P., Baccarani M., Deininger M.W., Cervantes F., Fujihara S., et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N. Engl. J. Med. 2017;376:917–927. doi: 10.1056/NEJMoa1609324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baccarani M., Deininger M.W., Rosti G., Hochhaus A., Soverini S., Apperley J.F., Cervantes F., Clark R.E., Cortes J.E., Guilhot F., et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosti G., Castagnetti F., Gugliotta G., Baccarani M. Tyrosine kinase inhibitors in chronic myeloid leukaemia: Which, when, for whom? Nat. Rev. Clin. Oncol. 2017;14:141–154. doi: 10.1038/nrclinonc.2016.139. [DOI] [PubMed] [Google Scholar]
- 12.Buffa P., Romano C., Pandini A., Massimino M., Tirro E., Di Raimondo F., Manzella L., Fraternali F., Vigneri P.G. BCR-ABL residues interacting with ponatinib are critical to preserve the tumorigenic potential of the oncoprotein. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2014;28:1221–1236. doi: 10.1096/fj.13-236992. [DOI] [PubMed] [Google Scholar]
- 13.Stagno F., Vigneri P., Consoli M.L., Cupri A., Stella S., Tambe L., Massimino M., Manzella L., Di Raimondo F. Hyperdiploidy associated with a high BCR-ABL transcript level may identify patients at risk of progression in chronic myeloid leukemia. Acta Haematol. 2012;127:7–9. doi: 10.1159/000330607. [DOI] [PubMed] [Google Scholar]
- 14.Jordanides N.E., Jorgensen H.G., Holyoake T.L., Mountford J.C. Functional ABCG2 is overexpressed on primary CML CD34+ cells and is inhibited by imatinib mesylate. Blood. 2006;108:1370–1373. doi: 10.1182/blood-2006-02-003145. [DOI] [PubMed] [Google Scholar]
- 15.Wagle M., Eiring A.M., Wongchenko M., Lu S., Guan Y., Wang Y., Lackner M., Amler L., Hampton G., Deininger M.W., et al. A role for FOXO1 in BCR-ABL1-independent tyrosine kinase inhibitor resistance in chronic myeloid leukemia. Leukemia. 2016;30:1493–1501. doi: 10.1038/leu.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massimino M., Stella S., Tirro E., Romano C., Pennisi M.S., Puma A., Manzella L., Zanghi A., Stagno F., Di Raimondo F., et al. Non ABL-directed inhibitors as alternative treatment strategies for chronic myeloid leukemia. Mol. Cancer. 2018;17:56. doi: 10.1186/s12943-018-0805-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishii Y., Nhiayi M.K., Tse E., Cheng J., Massimino M., Durden D.L., Vigneri P., Wang J.Y. Knockout Serum Replacement Promotes Cell Survival by Preventing BIM from Inducing Mitochondrial Cytochrome C Release. PLoS ONE. 2015;10:e0140585. doi: 10.1371/journal.pone.0140585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J., Yao X., Huang J. New tricks for human farnesyltransferase inhibitor: Cancer and beyond. Med. Chem. Comm. 2017;8:841–854. doi: 10.1039/C7MD00030H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sokal J.E., Cox E.B., Baccarani M., Tura S., Gomez G.A., Robertson J.E., Tso C.Y., Braun T.J., Clarkson B.D., Cervantes F., et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63:789–799. [PubMed] [Google Scholar]
- 20.Hasford J., Baccarani M., Hoffmann V., Guilhot J., Saussele S., Rosti G., Guilhot F., Porkka K., Ossenkoppele G., Lindoerfer D., et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: The EUTOS score. Blood. 2011;118:686–692. doi: 10.1182/blood-2010-12-319038. [DOI] [PubMed] [Google Scholar]
- 21.Pfirrmann M., Baccarani M., Saussele S., Guilhot J., Cervantes F., Ossenkoppele G., Hoffmann V.S., Castagnetti F., Hasford J., Hehlmann R., et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30:48–56. doi: 10.1038/leu.2015.261. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann V.S., Baccarani M., Lindoerfer D., Castagnetti F., Turkina A., Zaritsky A., Hellmann A., Prejzner W., Steegmann J.L., Mayer J., et al. The EUTOS prognostic score: Review and validation in 1288 patients with CML treated frontline with imatinib. Leukemia. 2013;27:2016–2022. doi: 10.1038/leu.2013.171. [DOI] [PubMed] [Google Scholar]
- 23.Marin D., Ibrahim A.R., Lucas C., Gerrard G., Wang L., Szydlo R.M., Clark R.E., Apperley J.F., Milojkovic D., Bua M., et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30:232–238. doi: 10.1200/JCO.2011.38.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanfstein B., Muller M.C., Hehlmann R., Erben P., Lauseker M., Fabarius A., Schnittger S., Haferlach C., Gohring G., Proetel U., et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML) Leukemia. 2012;26:2096–2102. doi: 10.1038/leu.2012.85. [DOI] [PubMed] [Google Scholar]
- 25.Vigneri P., Stagno F., Stella S., Cupri A., Forte S., Massimino M., Antolino A., Siragusa S., Mannina D., Impera S.S., et al. High BCR-ABL/GUS(IS) Levels at Diagnosis of Chronic Phase CML Are Associated with Unfavorable Responses to Standard-Dose Imatinib. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017;23:7189–7198. doi: 10.1158/1078-0432.CCR-17-0962. [DOI] [PubMed] [Google Scholar]
- 26.Bonecker S., Magnago M., Kaeda J., Solza C., Zalcberg Renault I. Is the BCR-ABL/GUSB transcript level at diagnosis an early predictive marker for chronic myeloid leukemia patients treated with imatinib? Rev. Bras. De Hematol. E Hemoter. 2015;37:142–143. doi: 10.1016/j.bjhh.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marin D., Hedgley C., Clark R.E., Apperley J., Foroni L., Milojkovic D., Pocock C., Goldman J.M., O’Brien S. Predictive value of early molecular response in patients with chronic myeloid leukemia treated with first-line dasatinib. Blood. 2012;120:291–294. doi: 10.1182/blood-2012-01-407486. [DOI] [PubMed] [Google Scholar]
- 28.Neelakantan P., Gerrard G., Lucas C., Milojkovic D., May P., Wang L., Paliompeis C., Bua M., Reid A., Rezvani K., et al. Combining BCR-ABL1 transcript levels at 3 and 6 months in chronic myeloid leukemia: Implications for early intervention strategies. Blood. 2013;121:2739–2742. doi: 10.1182/blood-2012-11-466037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim D.D., Hamad N., Lee H.G., Kamel-Reid S., Lipton J.H. BCR/ABL level at 6 months identifies good risk CML subgroup after failing early molecular response at 3 months following imatinib therapy for CML in chronic phase. Am. J. Hematol. 2014;89:626–632. doi: 10.1002/ajh.23707. [DOI] [PubMed] [Google Scholar]
- 30.Saussele S., Richter J., Hochhaus A., Mahon F.X. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. 2016;30:1638–1647. doi: 10.1038/leu.2016.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kantarjian H.M., Dixon D., Keating M.J., Talpaz M., Walters R.S., McCredie K.B., Freireich E.J. Characteristics of accelerated disease in chronic myelogenous leukemia. Cancer. 1988;61:1441–1446. doi: 10.1002/1097-0142(19880401)61:7<1441::AID-CNCR2820610727>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 32.Stagno F., Vigneri P., Del Fabro V., Stella S., Cupri A., Massimino M., Consoli C., Tambe L., Consoli M.L., Antolino A., et al. Influence of complex variant chromosomal translocations in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Acta Oncol. 2010;49:506–508. doi: 10.3109/02841861003660031. [DOI] [PubMed] [Google Scholar]
- 33.Muller M.C., Erben P., Saglio G., Gottardi E., Nyvold C.G., Schenk T., Ernst T., Lauber S., Kruth J., Hehlmann R., et al. Harmonization of BCR-ABL mRNA quantification using a uniform multifunctional control plasmid in 37 international laboratories. Leukemia. 2008;22:96–102. doi: 10.1038/sj.leu.2404983. [DOI] [PubMed] [Google Scholar]
- 34.Vella V., Puppin C., Damante G., Vigneri R., Sanfilippo M., Vigneri P., Tell G., Frasca F. DeltaNp73alpha inhibits PTEN expression in thyroid cancer cells. Int. J. Cancer. 2009;124:2539–2548. doi: 10.1002/ijc.24221. [DOI] [PubMed] [Google Scholar]
- 35.Hughes T., Deininger M., Hochhaus A., Branford S., Radich J., Kaeda J., Baccarani M., Cortes J., Cross N.C., Druker B.J., et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: Review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cross N.C., White H.E., Muller M.C., Saglio G., Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012;26:2172–2175. doi: 10.1038/leu.2012.104. [DOI] [PubMed] [Google Scholar]
- 37.Harrington D.P., Fleming T.R. A class of rank test procedures for censored survival data. Biometrika. 1982;69:553–566. doi: 10.1093/biomet/69.3.553. [DOI] [Google Scholar]
- 38.The R Project for Statistical Computing. [(accessed on 1 February 2019)]; Available online: https://www.r-project.org/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.