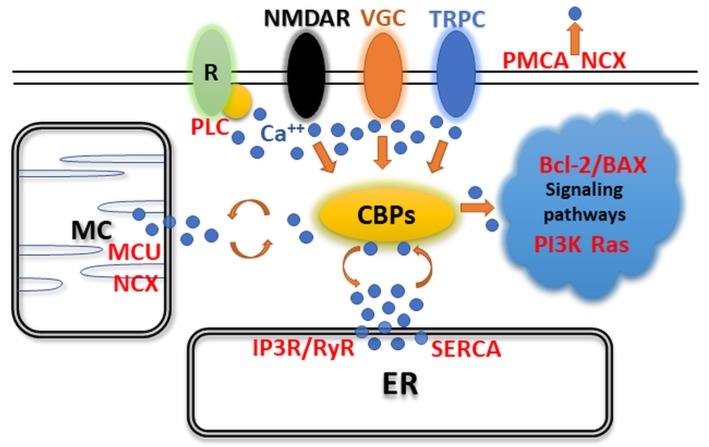
Figure 1.
Signaling of EF-hand Ca2+-binding buffer proteins (CaBPs) shows a central role in Ca2+ maintenance. Effects of G-protein-coupled phospholipase C (PLC), N-methyl-D-aspartate receptors (NMDARs), voltage-gated calcium channels (VGCs), and transient receptor potential cation channels (TRPCs) can be buffered by CaBPs. Intracellular Ca2+-dependent signal transduction is significantly controlled by CaBPs. This Ca2+ originates from mitochondria (MC) and endoplasmic reticulum (ER). Mitochondrial calcium uniporter (MCU), inositol trisphosphate receptor, ryanodine receptor (IP3R/RyR), and sarco and endoplasmic reticulum Ca2+-ATPase (SERCA) control Ca2+ levels intracellularly, while the sodium–calcium exchanger (NCX) can also be found on the MC and cell membranes. Plasma membrane Ca2+-ATPase (PMCA) and NCX are responsible for the removal of Ca2+ from the cell.