Abstract
Background:
Primary cutaneous lymphoma is a rare extranodal non-Hodgkin’s lymphoma confined to the skin. The data on the imaging findings of primary cutaneous lymphomas are largely lacking and the current diagnosis is based on clinical and histopathological examination. With the advances in dermatological ultrasound and molecular imaging, newer perspectives in the evaluation of cutaneous lymphomas are available.
Objective:
To review and describe the imaging findings in patient’s with the diagnosis of primary cutaneous lymphoma.
Methods:
A multicentric, retrospective observational study was undertaken in four countries to review the high resolution ultrasonography (HRUS) and fluorine 18-fludeoxyglucose positron emission tomography-computed tomography (PET-CT) imaging findings.
Results:
We had 41 patients, Female:Male 1:4.1; mean age, 57 years; range, 13–94 years. High resolution ultrasonography of the primary cutaneous lesions revealed thickening of the dermis in all the cases and the lesions were hypoechoic without any calcifications or central necrosis. The sonographic appearances of the lesions were categorised into focal infiltrative, nodular, pseudonodular, and diffusely infiltrative patterns. Nodular and pseudonodular lesions were predominant in B cell lymphomas, while diffusely infiltrative lesions were more common in T-cell lymphomas. On colour Doppler imaging, the lesions were hypervascular. Whole body 18F-fludeoxyglucose PET–CT imaging of the patients revealed increased uptake of the metabolite in the lesions.
Conclusion:
Sonographic patterns based on high resolution ultrasonography provide early clues to the non-invasive diagnosis of primary cutaneous lymphomas and PET-CT is the recommended modality of imaging for staging and follow-up.
Advances in knowledge:
High resolution ultrasound with colour Doppler and PET-CT imaging are complimentary to the clinical diagnosis of primary cutaneous lymphomas.
Introduction
Primary cutaneous lymphoma (PCL) is an uncommon form of extranodal non-Hodgkin’s lymphoma (NHL) characterised by malignant lymphocytes that are limited to the skin at initial diagnosis without any evidence of extracutaneous disease, i.e. lymph nodes, bone marrow or viscera at presentation and for 6 months after diagnosis, as assessed by adequate staging procedures.1–3 PCLs can arise from mature T lymphocytes, B lymphocytes or natural killer (NK) cells and are broadly classified as primary cutaneous T-cell lymphomas (PCTCL) and primary cutaneous B-cell lymphomas (PCBCL). Primary cutaneous lymphoma accounts for 19% of extranodal lymphomas with an estimated annual incidence of 1:100,000 and the approximate subtype incidence is about 65% for PCTCLs, 25% for PCBCLs, the rest being the NK cell neoplasms.1,3–5 The clinical manifestations of primary cutaneous lymphomas are completely dissimilar to those of secondary cutaneous lymphomas, i.e. nodal or systemic malignant lymphomas which secondarily involve the skin. The differentiation between these entities is of importance as both of them have entirely different therapeutic approach, prognosis, and treatment response.1,3 Despite the relative frequency of occurrence, primary cutaneous lymphomas are often missed by the clinicians and not considered in the differential diagnosis as they show considerable variation in the clinical features and immunohistological characteristics. As radiological investigations are rarely utilized in the evaluation of these neoplasms, very few studies and case reports that describe the imaging characteristics of these malignancies are available in literature. The purpose of this article is to describe the imaging features of primary cutaneous lymphomas to help in differentiating it from cutaneous neoplasms, metastases and other benign cutaneous lesions.
methods and materials
We undertook a multicentre, retrospective observational study of the imaging features of primary cutaneous lymphomas at our institutions over the previous 8 years between April 2010 and March 2018. This was undertaken in four countries, the four different centres being, Hyderabad (India), Santiago (Chile), Madrid (Spain), and Naples (Italy). The study was conducted by a group of physicians (both radiologists and dermatologists) having more than 20 years of working experience in the fields of dermatologic ultrasound and cutaneous malignancies. Electronic record databases were used to identify subjects with the diagnosis of primary cutaneous lymphomas, and wherever available, follow-up studies were reviewed. Inclusion criteria consisted of pathologically confirmed diagnosis of primary cutaneous lymphomas with abnormal imaging findings. Reactive lymphoid hyperplasias, i.e. cutaneous “pseudolymphomas” and pre-lymphomatous conditions which heal either spontaneously after elimination of the causative factor or after treatment with non-aggressive modalities are excluded from the study. The diagnosis of primary cutaneous lymphoma was established by histopathology and immunohistochemistry (IHC) of the tissue obtained by FNAC or open/excision biopsy. IHC was performed for both B-cell markers CD19 and CD20, and T-cell markers CD3 and CD5, to confirm the diagnosis. All the patients in this series initially underwent high resolution ultrasonography (HRUS) and colour Doppler examination of the lesions by using variable frequency high resolution transducers (15–22 MHz). The lesions were defined according to the sonographic appearance into four types (Figure 1) as follows: (1) “Focal infiltrative” lesions seen as irregular hypoechoic infiltrates within the dermis and subcutaneous tissue, (2) “Nodules” seen as hypoechoic nodules with a well demarcated border, (3) “Pseudonodules” seen as focal hypoechoic nodules with polylobulations, without a well demarcated border (4) “Diffusely infiltrative” lesions seen as hypoechoic, diffusely infiltrative, panniculitis like lesions involving the dermis and subcutaneous tissue. Later, in cases with multiple lesions, or for pre-treatment staging purpose, whole body 18F- FDG PET-CT imaging was performed.
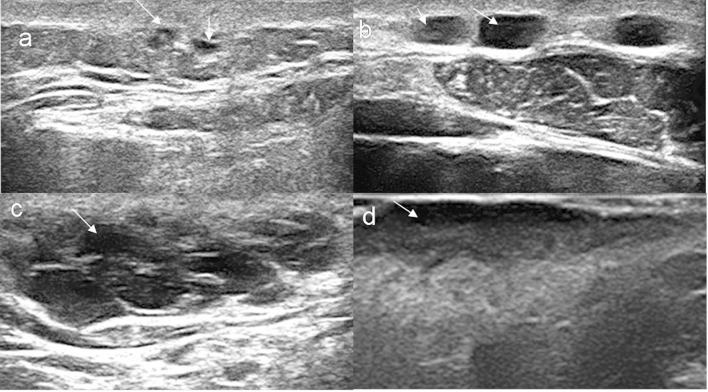
Figure 1.
Sonographic patterns of primary cutaneous lymphomas. (a) “Focal infiltrative” pattern seen as irregular hypoechoic infiltrates within the dermis and subcutaneous tissue (arrows). (b) “Nodular” pattern seen as hypoechoic nodules with well-defined borders (arrows). (c) “Pseudonodular” pattern seen as focal hypoechoic nodule with polylobulations and ill-defined borders (arrow). (d) “Diffusely infiltrative” pattern seen as hypoechoic, diffusely infiltrative, panniculitis like lesion (arrow).
Results
We retrospectively reviewed the imaging studies of 41 patients, (33 B cell and 8 T cell lymphomas) of primary cutaneous lymphomas. These neoplasms were seen more frequently in elderly patients with a male predominance (Female:Male 1:4.1; mean age, 57 years; range, 13–94 years). The clinical features and presentation ranged from plaques, papules, nodules to mixed cutaneous lesions, with reddish to violaceous discoloration of the overlying skin. B cell lymphomas were frequently seen as nodules, followed by plaques, whereas, T cell lymphomas presented as both cutaneous nodules and plaques, sometimes with ulceration. The lesions were solitary to multifocal, showing indolent to rapid growth, and the size of the lesions varied from 0.4 to 6.0 cm in diameter. The most common location of the lesions was head and neck, trunk, lower and upper limbs. Three patients had weight loss of about 4–11 kg with rapid increase in number, size and distribution the lesions.
Staging procedures used in the evaluation of the patients included physical examination, comprehensive blood examinations, chest X-ray, abdominal ultrasound, CT scans, bone marrow aspirates, bone scans and FDG PET scans. The follow-up time of the patients ranged from 1 month to 8 years.
Imaging findings
Chest X-ray and abdominal ultrasound did not reveal any abnormality. The cutaneous papules were seen on HRUS as focal infiltrative lesions and few of them coalesced later to form ill-defined pseudonodules (Figure 2). The cutaneous nodules were seen on HRUS as pseudonodules and nodules, the former being more common (Figure 3). The cutaneous plaques were seen on HRUS as hypoechoic, diffusely infiltrative lesions (Figure 4). The pseudonodular and nodular lesions were seen more frequently in B-cell lymphomas (70%), whereas, the diffusely infiltrative lesions were seen equally in both B cell and T cell lymphomas (50% each). HRUS revealed thickening of dermis in all the cases, and there was no evidence of necrosis, calcification or posterior acoustic shadowing in any of the lesions. On colour Doppler, the initial focal infiltrative lesions were avascular, but the nodular, pseudonodular, and diffusely infiltrative lesions were highly vascular (Figures 5 and 4c). CT scan of the thorax and abdomen showed the cutaneous nodules and plaque like lesions, but the papular lesions could not be clearly demarcated. There was no involvement of any other internal organs, lymph nodes or distant metastases. FDG PET scan was performed in 13 cases. The lesions showed increased uptake of FDG in 12 cases and one case of primary cutaneous B cell lymphoma did not show any FDG uptake. Patients were given chemotherapy and one patient of PCBCL refused treatment after diagnosis and staging. He later presented with extensive skeletal metastases and was treated with chemotherapy. Demographic data, anatomic areas of involvement, clinical, pathological and imaging features in patients are summarized in Table 1.
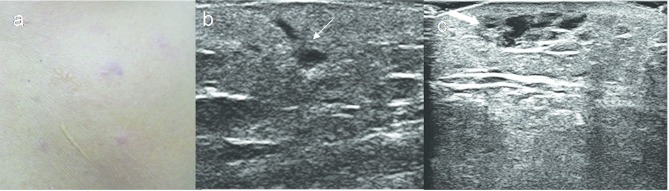
Figure 2.
A 52-year-old male with primary cutaneous T cell lymphoma. (a) Erythematous papules on the back. (b, c) HRUS images show focal infiltrative lesions (thin arrow) and small pseudonodule with thickening of dermis (thick arrow). HRUS, high resolution ultrasonography.
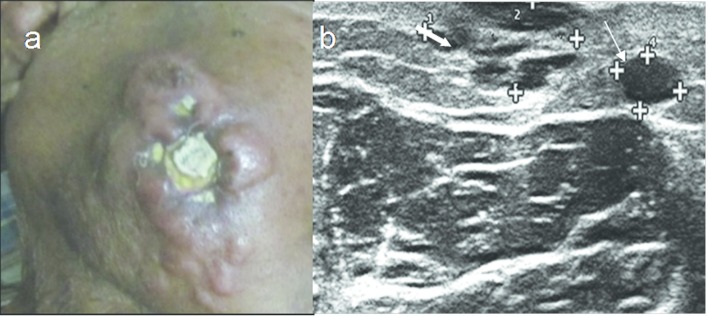
Figure 3.
A 74-year-old male with primary cutaneous B cell lymphoma. (a) Cutaneous nodules, ulceration and swelling on the cheek. (b) HRUS image shows nodular (thin arrow) and pseodonodular lesions (thick arrow). HRUS,high resolution ultrasonography.
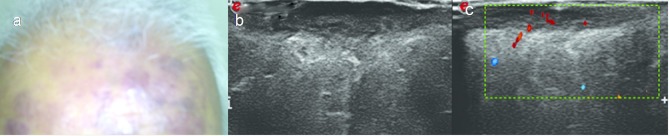
Figure 4.
A 60-year-old male with primary cutaneous T cell lymphoma. (a) Violaceous cutaneous plaques on forehead and scalp. (b) Split screen HRUS image shows hypoechoic diffusely infiltrative lesion with thickening of the dermis. (c) Colour Doppler image shows vascularity. HRUS, high resolution ultrasonography.
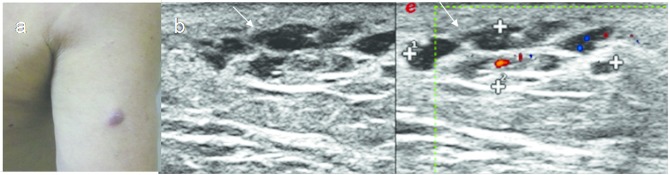
Figure 5.
A 59-year-old male with primary cutaneous B cell lymphoma. (a) Reddish nodule on the upper arm. (b) Split screen grey scale and colour Doppler HRUS images show hypoechoic lesion with ill-defined borders and internal vascularity—typical “pseudonodule” appearence. HRUS, high resolution ultrasonography.
Table 1.
Demographic, clinical, pathological and imaging features in patients (n = 41) with primary cutaneous lymphomas
| Subsection | B Cell | T Cell | |
| Histopathology | 33 (80.4%) | 8 (19.6%) | |
| Sex | |||
| Male | 23 (56.0%) | 4 (9.7%) | |
| Female | 10 (24.4%) | 4 (9.7%) | |
| Race | |||
| Caucasian | 30 (73.1%) | 8 (19.6%) | |
| Mediterranean | 3 (7.3%) | 0 | |
| Location | |||
| Head and neck | 11 (26.8%) | 1 (2.4%) | |
| Chest/thorax | 5 (12.2%) | 1 (2.4%) | |
| Abdomen and genitals | 1 (2.4%) | 2 (4.8%) | |
| Upper limbs | 3 (7.3%) | 1 (2.4%) | |
| Lower limbs | 5 (12.2%) | 3 (7.31%) | |
| Upper back | 1 (2.4%) | 0 | |
| Lower back and buttocks | 1 (2.4%) | 0 | |
| Multiple sites | 6 (14.6%) | 0 | |
| Clinical features | |||
| Nodules | 18 (43.9%) | 3 (7.31%) | |
| Papules | 2 (4.87%) | 1 (2.4%) | |
| Plaques | 8 (19.6%) | 4 (9.7%) | |
| Mixed lesions (nodules and plaques) | 5 (12.2%) | 0 | |
| Growth | |||
| Rapid growth | 3 (7.3%) | 0 | |
| Indolent slow growth | 30 (73.1%) | 8 (19.6%) | |
| Ultrasound features | |||
| Early focal infiltrative lesions | 26 (63.4%) | 0 | |
| Nodular lesions | 15 (36.6%) | 2 (4.8%) | |
| Pseudonodular lesions | 19 (36.6%) | 2 (4.8%) | |
| Diffuse infiltrative lesions | 10 (24.4%) | 4 (9.7%) | |
| PET-CT | |||
| Total cases PET/CT performed | 9 | 4 | |
| Avid uptake by cutaneous lesions | 8 (61.5%) | 4 (30.7%) | |
| No uptake by cutaneous lesions | 1 (7.7%) | 0 | |
| Lymph nodes | 0 | 0 | |
| Distant metastases | 1 (2.4%) | 0 | |
PET, positron emission tomography.
Discussion
The term primary cutaneous lymphoma refers to the NHLs involving the skin, without any evidence of extracutaneous involvement at the time of diagnosis and initial staging evaluation.1,6 The skin is the second most common site affected in primary extranodal NHL whereas cutaneous involvement is rare in patients with Hodgkin disease.1,4,7 Despite the morphological similarity, considerable difference exists between primary and secondary cutaneous lymphomas, as PCLs have an indolent course and better prognosis compared to the secondary cutaneous lymphomas.1,7,8
PCLs are usually seen in middle aged to elderly patients, and they have a slight male preponderance.9,10 In a recent study by Lee et al, it was found that the relative frequency of PCBCL has notably increased in the last two decades, and also it occurred in significantly older population compared to PCTCL.10 PCLs are variable in presentation and are seen as red to violaceous patches, papules and skin nodules without scaling or ulceration, except in T cell lymphomas which can present with ulceration and cellulitis-like swelling.10 The lesions can be solitary or multiple, usually confined to one body area, with multiple skin lesions being more common in PCTCL (90%) than PCBCL (50%).9,10 The commonest location of PCTCL lesions is the trunk, followed by the leg, arm and head and neck areas, whereas, PCBCLs are most commonly seen in the head and neck regions.10
The neoplastic cells express specific cluster of designation (CD) markers that are used to characterize the cells and lineage, hence IHC is crucial in the diagnosis and classification of cutaneous lymphomas.8,11
Though HRUS with colour Doppler using high, variable frequency transducers is being increasingly used in the imaging of cutaneous lesions, the data on imaging of cutaneous lymphomas are very scarce in published radiological literature.12,13 Giovagnorio, in 1997, has first described the sonographic features of primary cutaneous lymphomas, and stated that B-cell lymphomas produced sharply demarcated infiltrates in a “focal nodular” pattern and T-cell lymphomas caused diffuse dermal involvement.12 The present data show that majority of B cell lymphomas revealed hypoechoic, polylobulated pattern with pseudonodular appearance and T cell lymphomas revealed hypoechoic, diffusely infiltrative lesions, but these patterns are not mutually exclusive, and the sonographic pattern does not always correlate with the histological diagnosis as suggested by “Giovagnorio”. The other sonographic features observed consistently in all our cases were thickening of dermis, absence of calcifications and central necrosis. In the early stages, the cutaneous papules and small nodular lesions appeared as focal hypoechoic infiltrations in the dermis and were avascular on colour Doppler but the nodular, pseudonodular and diffuse infiltrative lesions were vascular. This indicates a close relationship between growth of the nodules and development of internal vascularity, as vascularity suggests the malignant nature of the cutaneous lesions.14 Chiou et al in their study of ultrasonography of primary peripheral soft tissue lymphomas, have observed that they were extremely vascular compared to other peripheral soft tissue tumours.15 We have noticed similar hypervascularity in primary cutaneous lymphomas.
Primary cutaneous lymphomas are frequently multifocal and physical examination largely underestimates the disease burden.5,6 Although, HRUS can show the sonographic features and the extent of the infiltration of the lesions in the cutaneous layers, it does not show the progression of the disease beyond the primary superficial lesion. Another limitation of HRUS is that, it only suggests the diagnosis and guides the biopsy but does not confirm it.
Systemic dissemination significantly alters the prognosis and therapeutic approach in PCL, therefore it is imperative to accurately stage and define the disease with other imaging modalities.16,17
The role of cross-sectional imaging in cutaneous lymphomas using CT and MRI is not well-documented as the lesions are too small and superficial to be adequately imaged and characterised. CT and MRI may be useful to some extent in assessing the exact size of the lesion, demonstrating regional multifocality and local staging of the tumour.18,19
The detection of disease with FDG-PET is based on metabolism. PET alone has poor anatomical resolution, but FDG PET and CT, together, has proved superior to CT in the staging, restaging and extranodal detection of lymphomas.5,20,21 PET-CT is the modality of choice in detecting extracutaneous involvement, monitoring the post-therapeutic disease activity and is routinely recommended for the staging of patients with FDG-avid, potentially curable lymphomas.5,6 PET-CT imaging has revealed increased up take of FDG in the cutaneous lesions in 92.3% of the cases in our study (Figures 6–8). We have, in our series, one case of PCBCL revealing extensive skeletal metastases on PET-CT, and review of literature has not revealed any other case with skeletal metastases (Figure 9).
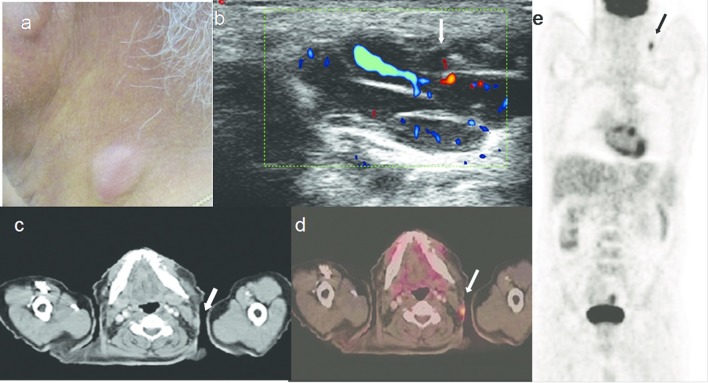
Figure 6.
A 63-year-old male with primary cutaneous B cell lymphoma. (a). Reddish nodule on the right side-of neck. (b) HRUS with colour Doppler image shows polylobulated nodule with vascularity. (c, d, e.) Axial CT, PET-CT fusion and whole body FDG PET scan images show the subcutaneous nodule in the neck with FDG uptake (arrows). FDG, fludeoxyglucose; HRUS, high resolution ultrasonography; PET, positron emission tomography.
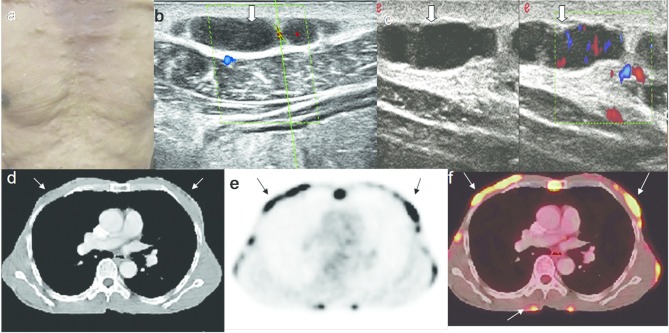
Figure 7.
An 81-year-old male with primary cutaneous B cell lymphoma. (a) Multiple cutaneous nodules on the chest. (b, c.) Grey scale and colour Doppler HRUS images show inhomogeneous, hypoechoic nodules with vascularity. (d) Axial CT scan image shows the small lesions in the chest wall, which are poorly differentiated from the surrounding soft tissues. (e). Axial FDG PET scan image shows the FDG uptake in multiple subcutaneous nodules. (f) Axial PET-CT fusion image shows the cutaneous nodules with excellent anatomic resolution. FDG, fludeoxyglucose; HRUS, high resolution ultrasonography; PET, positron emission tomography.
Figure 8.
A 67-year-old male with primary cutaneous T cell lymphoma.(a). Ulcerated plaque on the abdominal wall. (b). HRUS images shows hypoechoic diffusely infiltrative lesion with thickening of the dermis. (c, d, & e). Axial CT, FDG PET and PET-CT fusion images show the lesion with FDG uptake (arrows). FDG, fludeoxyglucose; HRUS, high resolution ultrasonography; PET, positron emission tomography.
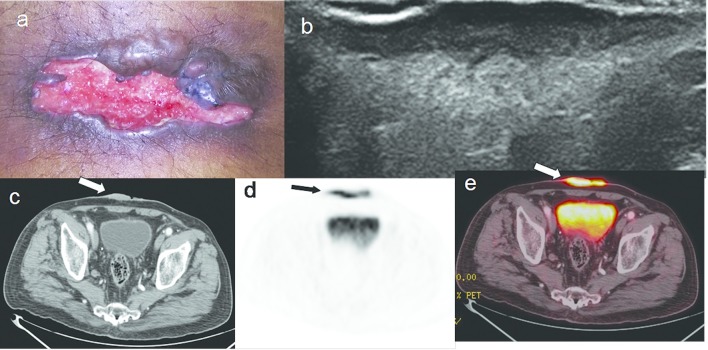
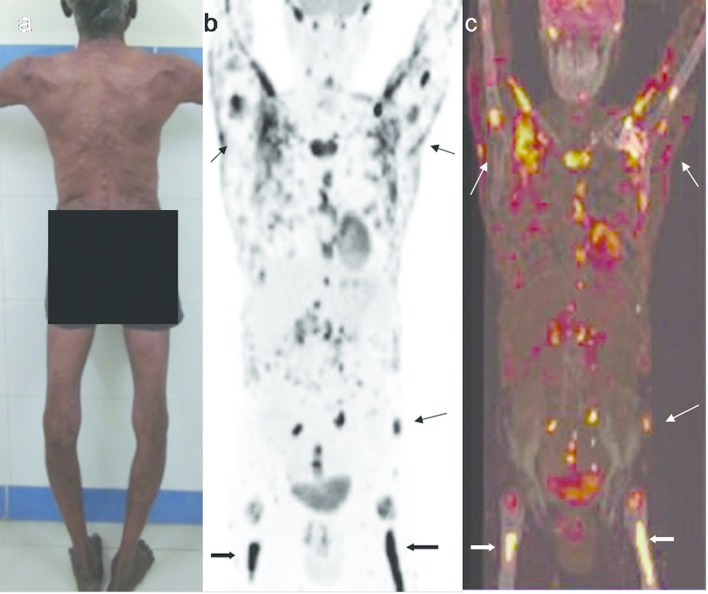
Figure 9.
A 57-year-old male with primary cutaneous B cell lymphoma having extensive skeletal metastases. (a) Multiple cutaneous nodules on trunk, back, abdomen, upper and lower limbs. (b, c). Whole body FDG PET scan and PET-CT fusion images show FDG uptake in cutaneous nodules (thin arrows), and in axial and appendicular skeleton (thick arrows). FDG, fludeoxyglucose; HRUS, high resolution ultrasonography; PET, positron emission tomography.
The International Society for Cutaneous Lymphomas and European Organization for Research and Treatment of Cancer have proposed a TNM (tumour, node, metastases) system to document the anatomic extent of disease and have recommended guidelines and staging evaluations for appropriate and effective management of cutaneous lymphomas.6,16,18 Apart from this, the outcome and prognosis in all subtypes of non- Hodgkin lymphomas is being predicted using the International Prognostic Index, which considers patients age, serum LDH, location and extent of skin involvement, and other markers in predicting the prognosis.17,22 Currently, only CT, whole-body PET (1 8F-FDG) and ultrasound of neck are included in the International Society for Cutaneous Lymphomas–European Organization for Research and Treatment of Cancer guidelines for evaluation of PCL, and HRUS of the primary lesions has no role either in the diagnostic evaluation or in the prognostic index. Recent studies show that high frequency ultrasound is being used effectively in monitoring the therapeutic response to brachytherapy in cutaneous malignancies.23 Polańska et al have successfully used HRUS in the evaluation and assessment of skin lesions in Mycosis fungoides, the most common subtype of primary cutaneous T-cell lymphoma.24 In addition to diagnosis, HRUS can also be utilised in estimating the response to treatment in cutaneous lymphomas as it provides non-invasive, repeatable, quantitative assessment of the lesions. With the recent advances in transducer technology and expanding indications of ultrasonography in the evaluation of dermatological lesions, we envisage increasing usage of HRUS in the evaluation of primary lesions and monitoring the course of cutaneous lymphomas.
Conclusion
The clinical, histopathological and imaging features of primary cutaneous lymphomas are different from other cutaneous malignancies. This review on the imaging features is intended to provide an overview of the utility and limitations of various imaging modalities in the evaluation of primary cutaneous lymphomas. The authors expect that newer data continue to evolve, and in future, imaging plays an important role in early and improved non-invasive diagnosis of primary cutaneous lymphomas.
Footnotes
Ethics approval: Approved by the Institutional Ethics Committee, Basavatarakam Indo American Cancer Hospital & Research Institute, Reference No:IEC/2018/117. Guarantor: Dr. Anitha Mandava.
Contributor Information
Anitha Mandava, Email: dranitha96@gmail.com.
Veeraiah Koppula, Email: veeraiah_koppula@yahoo.com.
Ximena Wortsman, Email: xworts@yahoo.co.
Orlando Catalano, Email: orlando.catalano@istitutovarelli.it.
Fernando Alfageme, Email: dermalfageme@gmail.com.
REFERENCES
- 1. Willemze R , Jaffe ES , Burg G , Cerroni L , Berti E , Swerdlow SH , et al. . WHO-EORTC classification for cutaneous lymphomas . Blood 2005. ; 105 : 3768 – 85 . doi: 10.1182/blood-2004-09-3502 [DOI] [PubMed] [Google Scholar]
- 2. Pandolfino TL , Siegel RS , Kuzel TM , Rosen ST , Guitart J . Primary cutaneous B-cell lymphoma: review and current concepts . J Clin Oncol 2000. ; 18 : 2152 – 68 . doi: 10.1200/JCO.2000.18.10.2152 [DOI] [PubMed] [Google Scholar]
- 3. Sokołowska-Wojdyło M , Olek-Hrab K , Ruckemann-Dziurdzińska K . Primary cutaneous lymphomas: diagnosis and treatment . Advances in Dermatology and Allergology 2015. ; 5 : 368 – 83 . doi: 10.5114/pdia.2015.54749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bradford PT , Devesa SS , Anderson WF , Toro JR . Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases . Blood 2009. ; 113 : 5064 – 73 . doi: 10.1182/blood-2008-10-184168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fardin S , Gholami TJ , Werner AH , Rook AA . Imaging evaluation of cutaneous lymphoma using functional and structural imaging . In Imaging in Dermatology 2016. ;: 485 – 90 . [Google Scholar]
- 6. Kim YH , Willemze R , Pimpinelli N , Whittaker S , Olsen EA , Ranki A , et al. . TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC . Blood 2007. ; 110 : 479 – 84 . doi: 10.1182/blood-2006-10-054601 [DOI] [PubMed] [Google Scholar]
- 7. Paes FM , Kalkanis DG , Sideras PA , Serafini AN . FDG PET/CT of extranodal involvement in non-Hodgkin lymphoma and Hodgkin disease . Radiographics 2010. ; 30 : 269 – 91 . doi: 10.1148/rg.301095088 [DOI] [PubMed] [Google Scholar]
- 8. Sousa ARD , Costa IS , Araujo Filho EF , Juca NBH , Miranda WLL . Primary cutaneous large B-cell lymphoma of atypical presentation: a case report . An Bras Dermatol 2011. ; 86 : 549 – 51 . [DOI] [PubMed] [Google Scholar]
- 9. Hembury TA , Lee B , Gascoyne RD , Macpherson N , Yang B , House N , et al. . Primary cutaneous diffuse large B-cell lymphoma: a clinicopathologic study of 15 cases . Am J Clin Pathol 2002. ; 117 : 574 – 80 . doi: 10.1309/9DAQ-PWP3-XKDG-CUTG [DOI] [PubMed] [Google Scholar]
- 10. Lee WJ , Lee SH , Moon IJ , Won CH , Chang SE , Choi JH , et al. . Relative frequency, clinical features, and survival outcomes of 395 patients with cutaneous lymphoma in Korea: A subgroup analysis per 10-year period . Acta Derm Venereol 2016. ; 96 : 888 – 93 . doi: 10.2340/00015555-2404 [DOI] [PubMed] [Google Scholar]
- 11. Santucci M , Pimpinelli N , Arganini L . Primary cutaneous B-cell lymphoma: a unique type of low-grade lymphoma. Clinicopathologic and immunologic study of 83 cases . Cancer 1991. ; 67 : 2311 – 26 . doi: [DOI] [PubMed] [Google Scholar]
- 12. Giovagnorio F . Sonography of cutaneous non-Hodgkin's lymphomas . Clin Radiol 1997. ; 52 : 301 – 3 . doi: 10.1016/S0009-9260(97)80059-1 [DOI] [PubMed] [Google Scholar]
- 13. Rodríguez Bandera AI , Moreno Bonilla G , Feito Rodríguez M , Beato Merino MJ , de Lucas Laguna R . Usefulness of high-frequency ultrasonography in the assessment of cutaneous lesions in children with hematologic malignancies . Pediatr Dermatol 2018. ; 35 : e276 – e280 . doi: 10.1111/pde.13563 [DOI] [PubMed] [Google Scholar]
- 14. Szymańska E , Nowicki A , Mlosek K , Litniewski J , Lewandowski M , Secomski W , et al. . Skin imaging with high frequency ultrasound - preliminary results . Eur J Ultrasound 2000. ; 12 : 9 – 16 . doi: 10.1016/S0929-8266(00)00097-5 [DOI] [PubMed] [Google Scholar]
- 15. Chiou HJ , Chou YH , Chiou SY , Chen WM , Chen W , Wang HK , et al. . High-resolution ultrasonography of primary peripheral soft tissue lymphoma . J Ultrasound Med 2005. ; 24 : 77 – 86 . doi: 10.7863/jum.2005.24.1.77 [DOI] [PubMed] [Google Scholar]
- 16. Willemze R , Hodak E , Zinzani PL , Specht L , Ladetto M , . ESMO Guidelines Working Group . Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up . Ann Oncol 2013. ; 24 Suppl 6 : vi149 – vi154 . doi: 10.1093/annonc/mdt242 [DOI] [PubMed] [Google Scholar]
- 17. Lee HJ , Im JG , Goo JM , Kim KW , Choi BI , Chang KH , et al. . Peripheral T-cell lymphoma: spectrum of imaging findings with clinical and pathologic features . Radiographics 2003. ; 23 : 7 – 26 . doi: 10.1148/rg.231025018 [DOI] [PubMed] [Google Scholar]
- 18. Kumar R , Xiu Y , Zhuang HM , Alavi A . 18F-fluorodeoxyglucose-positron emission tomography in evaluation of primary cutaneous lymphoma . Br J Dermatol 2006. ; 155 : 357 – 63 . doi: 10.1111/j.1365-2133.2006.07367.x [DOI] [PubMed] [Google Scholar]
- 19. Vanhoenacker FM , Baten A , Vandeputte V . Imaging findings of a cutaneous B-cell lymphoma . Jbr–btr 2009. ; 92 : 285 – 8 . [PubMed] [Google Scholar]
- 20. Seam P , Juweid ME , Cheson BD . The role of FDG-PET scans in patients with lymphoma . Blood 2007. ; 110 : 3507 – 16 . doi: 10.1182/blood-2007-06-097238 [DOI] [PubMed] [Google Scholar]
- 21. Schaefer NG , Hany TF , Taverna C , Seifert B , Stumpe KD , von Schulthess GK , et al. . Non-Hodgkin lymphoma and Hodgkin disease: coregistered FDG PET and CT at staging and restaging--do we need contrast-enhanced CT? Radiology 2004. ; 232 : 823 – 9 . doi: 10.1148/radiol.2323030985 [DOI] [PubMed] [Google Scholar]
- 22. Freedman AS , Nadler LM . Malignancies of lymphoid cells . : Fauci AS, Braunwald E, Isselbacher KJ . 25 . New York, NY: : The British Institute of Radiology. ; 2001. . [Google Scholar]
- 23. Ballester-Sánchez R , Pons-Llanas O , Llavador-Ros M , Botella-Estrada R , Ballester-Cuñat A , Tormo-Micó A , et al. . Depth determination of skin cancers treated with superficial brachytherapy: ultrasound vs. histopathology . J Contemp Brachytherapy 2015. ; 6 : 356 – 61 . doi: 10.5114/jcb.2014.47860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Polańska A , Dańczak-Pazdrowska A , Olek-Hrab K , Osmola-Mańkowska A , Bowszyc-Dmochowska M , Żaba R , et al. . High-frequency ultrasonography-New non-invasive method in assessment of skin lymphomas . Skin Res Technol 2018. ; 24 : 517 – 21 . doi: 10.1111/srt.12450 [DOI] [PubMed] [Google Scholar]