Abstract
Background
Malaria control activities can have a disproportionately greater impact on Plasmodium falciparum than on P. vivax in areas where both species are coendemic. We investigated temporal trends in malaria-related morbidity and mortality in Papua, Indonesia, before and after introduction of a universal, artemisinin-based antimalarial treatment strategy for all Plasmodium species.
Methods and findings
A prospective, district-wide malariometric surveillance system was established in April 2004 to record all cases of malaria at community clinics and the regional hospital and maintained until December 2013. In March 2006, antimalarial treatment policy was changed to artemisinin combination therapy for uncomplicated malaria and intravenous artesunate for severe malaria due to any Plasmodium species. Over the study period, a total of 418,238 patients presented to the surveillance facilities with malaria. The proportion of patients with malaria requiring admission to hospital fell from 26.9% (7,745/28,789) in the pre–policy change period (April 2004 to March 2006) to 14.0% (4,786/34,117) in the late transition period (April 2008 to December 2009), a difference of −12.9% (95% confidence interval [CI] −13.5% to −12.2%). There was a significant fall in the mortality of patients presenting to the hospital with P. falciparum malaria (0.53% [100/18,965] versus 0.32% [57/17,691]; difference = −0.21% [95% CI −0.34 to −0.07]) but not in patients with P. vivax malaria (0.28% [21/7,545] versus 0.23% [28/12,397]; difference = −0.05% [95% CI −0.20 to 0.09]). Between the same periods, the overall proportion of malaria due to P. vivax rose from 44.1% (30,444/69,098) to 53.3% (29,934/56,125) in the community clinics and from 32.4% (9,325/28,789) to 44.1% (15,035/34,117) at the hospital. After controlling for population growth and changes in treatment-seeking behaviour, the incidence of P. falciparum malaria fell from 511 to 249 per 1,000 person-years (py) (incidence rate ratio [IRR] = 0.49 [95% CI 0.48–0.49]), whereas the incidence of P. vivax malaria fell from 331 to 239 per 1,000 py (IRR = 0.72 [95% CI 0.71–0.73]). The main limitations of our study were possible confounding from changes in healthcare provision, a growing population, and significant shifts in treatment-seeking behaviour following implementation of a new antimalarial policy.
Conclusions
In this area with high levels of antimalarial drug resistance, adoption of a universal policy of efficacious artemisinin-based therapy for malaria infections due to any Plasmodium species was associated with a significant reduction in total malaria-attributable morbidity and mortality. The burden of P. falciparum malaria was reduced to a greater extent than that of P. vivax malaria. In coendemic regions, the timely elimination of malaria will require that safe and effective radical cure of both the blood and liver stages of the parasite is widely available for all patients at risk of malaria.
Ric Price and colleagues report on changes in Plasmodium falciparum and vivax malaria following a universal malaria treatment programme in Papua, Indonesia.
Author summary
Why was this study done?
Multidrug-resistant malaria results in recurrent parasitaemia, a cumulative risk of anaemia, and progression to severe and fatal disease.
Whilst artemisinin combination therapies (ACTs) and intravenous (IV) artesunate can reduce morbidity and mortality associated with P. falciparum malaria, they have no activity on the hypnozoite stages of P. vivax, which can relapse weeks to months following an initial infection and sustain ongoing transmission of the parasite.
In Papua, Indonesia, antimalarial resistance has emerged in both P. falciparum and P. vivax. We used a prospective malariometric surveillance network to investigate the differential impact of a universal policy of ACTs for uncomplicated malaria and IV artesunate for severe malaria on the morbidity and mortality attributable to P. falciparum and P. vivax.
What did the researchers do and find?
After controlling for population growth and changes in treatment-seeking behaviour, the incidence of malaria after policy change fell by about 60% for P. falciparum and 40% for P. vivax.
There was a 50% fall in the proportion of patients with malaria requiring admission to hospital and a 30% fall in malaria-related mortality. Bed occupancy due to admission with malaria fell by 25%.
Whilst there was a small decrease in the absolute incidence of P. vivax infections over the 9-year study period, the proportion of malaria cases and malaria-attributable deaths to P. vivax increased with time.
What do these findings mean?
The results highlight the importance of highly effective blood schizontocidal antimalarial drugs in reducing the overall burden of drug-resistant malaria.
The rising proportion of malaria due to P. vivax emphasizes the need for safe and effective drug regimens that clear both the blood and liver stages of P. vivax in malaria elimination efforts in coendemic regions.
Introduction
Prompt and effective treatment of malaria reduces morbidity and limits onward transmission of the Plasmodium parasite [1,2]. Large-scale use of highly efficacious antimalarial treatment regimens has contributed to significant reductions in P. falciparum malaria in many malaria-endemic regions [3,4]. P. vivax is more difficult to cure than P. falciparum because it forms dormant liver stages (hypnozoites) that are intrinsically resistant to standard schizontocidal drugs. Unless patients are treated with an effective drug regimen that clears both the blood and liver stage of the parasite, these hypnozoites can reactivate periodically, causing recurrent blood-stage infections (relapses) and ongoing transmission [5]. Malaria treatment campaigns that do not include radically curative primaquine regimens for patients infected with P. vivax may have only a modest effect on the number of cases of P. vivax malaria and thus are likely to be associated with an increase in the proportion of malaria due to this parasite compared to P. falciparum [6–8]. When primaquine radical cure is included in national guidelines, it is usually prescribed without prior testing for glucose-6-phosphate dehydrogenase (G6PD) deficiency. To mitigate the risks of drug-induced haemolysis, many countries recommend a 15-mg daily dose administered over 14 days despite evidence showing that 30 mg daily is more effective [9]. When supervised, a 14-day regimen of primaquine can reduce the risk of P. vivax relapse by more than 85% [10,11]; however, in most endemic settings, daily supervision of such a prolonged treatment regimen is not practical [12], and this can result in a significant reduction in primaquine adherence and effectiveness [13–15]. In a large-scale observational study of patients with vivax malaria in Papua, Indonesia, the effectiveness of unsupervised primaquine was estimated to be only 10% [16].
Malaria endemicity in Papua, Indonesia, varies from hypo- to hyperendemic for P. falciparum and P. vivax [17]. In the early 2000s, clinical trials and ex vivo drug-susceptibility testing demonstrated high-grade resistance to chloroquine and sulphadoxine + pyrimethamine in endemic P. falciparum strains and chloroquine resistance in P. vivax strains [18–20]. Frequent, recurrent parasitaemia is more likely in the setting of high-grade drug resistance and is associated with a cumulative risk of chronic anaemia, severe malaria, and mortality [21,22]. Therefore, in March 2006, Indonesian national antimalarial treatment guidelines were changed to an artemisinin combination therapy (ACT) (dihydroartemisinin plus piperaquine [DP]) for uncomplicated malaria due to any Plasmodium species and intravenous (IV) artesunate for severe malaria. At the same time, policy for the use of primaquine in patients with P. vivax infections was changed from a total dose of 3.5 mg/kg over 14 days to a higher dose of 7 mg/kg over 14 days. Information regarding the treatment changes was distributed widely via health professionals and community leaders. In Mimika District, located in southern Papua Province, hospital and community surveillance systems were put into place prior to the policy change to allow an assessment of the subsequent changes in malaria-attributable morbidity and mortality due to either P. falciparum or P. vivax. Previous analyses from the same location have quantified the burden of malaria in the hospital, the epidemiology of malaria in the community, and local treatment-seeking behaviour [17,22,23]. The current analysis used routinely collected surveillance data collected over a 9-year period (2004 to 2013) to investigate the temporal trends in malaria morbidity and mortality before and after the change in antimalarial treatment policy and the relative impact of this intervention on the burden of P. falciparum compared with P. vivax.
Methods
Study site
The geography, climate, and demographics of Mimika District and its capital, Timika, have been described previously [17,22]. Briefly, Mimika District lies in south central Papua, eastern Indonesia, and covers an area of 21,522 km2; it has 12 subdistricts and 85 villages (Fig 1). The region has fragmented forest ranging from extensive coastal lowlands to high mountainous environments. Timika has a growing population of native Papuans and Indonesian migrants, estimated to be 120,457 in 2004 and increasing to 196,401 in 2013 [24].
Fig 1. Map of Mimika district in Papua Province, eastern Indonesia.
Map adapted from WorldOfMaps (https://www.worldofmaps.com).
Malaria transmission is perennial with minimal seasonal variation and is normally restricted to the lowland areas below 1,600-m elevation, where most of the population now resides. There are three primary mosquito vectors: Anopheles koliensis, two members of the A. farauti complex, and A. punctulatus; all of these vectors are both exo- and endophilic and are primarily opportunistic in host-seeking behaviour. In 2005, the point prevalence of parasitaemia was estimated to be 16.3%: 46% due to P. falciparum, 39% due to P. vivax, and 11% due to mixed P. falciparum/P. vivax infections [17]. Local P. vivax strains have a typical equatorial relapse periodicity of 3–4 weeks [16,25].
Clinical trials conducted in Timika in 2004 and 2005 demonstrated failure of chloroquine and sulphadoxine + pyrimethamine for the treatment of uncomplicated falciparum malaria with a recurrence rate following combination treatment of 48% at day 28 [18]. Recurrence of P. vivax at day 28 post chloroquine monotherapy was even higher at 65% [18]. Ex vivo studies confirmed high-grade resistance to these drugs [19].
Health facilities
Formal healthcare facilities in the district include Rumah Sakit Mitra Masyarakat (RSMM), a 110-bed hospital funded by a local mining company providing healthcare free of charge to indigenous Papuan communities and at a nominal cost to non-Papuan Indonesians. Additionally, there are 12 government-funded community primary health clinics (puskesmas) and 10 clinics administered by the local mining company. A government-funded hospital (Rumah Sakit Umum Daerah [RSUD]) opened at the end of 2008 but did not begin seeing substantial numbers of malaria patients until 2010. Antimalarial treatment can also be bought at a wide range of regulated and unregulated private sector clinics and facilities in Timika [23].
Antimalarial treatment policy change
During the early 2000s, patients with uncomplicated malaria presenting to community clinics were treated with chloroquine plus sulphadoxine + pyrimethamine if they had falciparum malaria and with chloroquine plus low-dose primaquine (total dose 3.5 mg/kg) if they had vivax malaria. At the RSMM hospital, patients with uncomplicated malaria were treated with oral quinine, whereas patients with severe malaria received IV quinine. Those with P. vivax malaria also received unsupervised low-dose primaquine [16]. In March 2006, the policy for treatment of uncomplicated malaria due to any Plasmodium species at all public community clinics and the hospital was changed to DP, a regimen with a risk of P. falciparum recrudescence of 4.4% and P. vivax recurrence of 10% by day 42 [26]. At the same time, treatment of severe malaria was changed from IV quinine to IV artesunate [27], and the dose of unsupervised primaquine was doubled to 7 mg/kg divided over 14 days. The new unified antimalarial treatment policy was adopted by RSUD hospital upon opening. Healthcare providers disseminated information regarding the antimalarial policy change and the benefits of DP to clinics and to communities via village leaders.
Community surveillance
All patients presenting to one of the formal sector community (puskesmas) clinics with symptoms consistent with malaria had capillary blood collected for blood film examination or had a rapid diagnostic test prior to antimalarial treatment. Between April 2004 and December 2009, weekly reports on the number of blood film examinations and the number of patients treated for malaria were collated by the district health authority. These reports were aggregated by the species of infection within four age bands: <1 year, 1 to 5 years, 5 to 10 years, and those older. Malariometric surveillance data were not collected from healthcare facilities in the private sector.
Cluster-randomized, cross-sectional surveys to determine treatment-seeking behaviour were conducted in 2005 and again in 2013, using an identical sampling strategy [17,23]. In 2005, during the pre–policy change period, 45.7% (349/764) of patients with malaria presented to public sector facilities and would have been detected by the surveillance network, but in 2013, 6 years after policy change, this figure had risen to 67.3% (66/98; p < 0.001) [28]. The shifts in treatment-seeking behaviour were apparent in all age groups. In the first household survey, 32.3% (10/31) of members who died of any cause within the preceding year did so at RSMM hospital compared to 26.3% (5/19) in 2013 (p = 0.656).
Hospital surveillance
All patient presentations to RSMM between 2004 and 2013 (whether to the outpatients department, emergency department, or inpatient wards) were recorded by hospital administrators in a QPro database. Patients were identified using a unique hospital reference number. Demographic data and the clinical diagnoses assigned by the attending physician were collected. Drug prescriptions and results of full blood-count analyses from RSMM’s Coulter counter were recorded in separate databases and identified by the same individual hospital reference number. RSMM policy dictates that all outpatients with fever or other signs or symptoms consistent with malaria and all inpatients regardless of presentation have a thick film prepared for malaria microscopy. Thin films and rapid diagnostic tests were done after-hours when the laboratory was closed. At RSUD hospital, a malaria register was initiated in January 2010, documenting aggregated data on basic demographic details, infecting Plasmodium species, fulfilment of clinical criteria for severe malaria, admission status, and malaria-related death in patients at the hospital.
Entomology and meteorology
Since 2002, vector-control activities in the region have been provided predominantly by the Public Health Malaria Control (PHMC) programme. These have included twice-yearly indoor residual spraying (IRS) and distribution of long-lasting insecticide-treated bed nets (LLINs) covering 10%–20% of households. Vector-control activities remained relatively constant from 2004 until mid-2013, when a large-scale IRS campaign and bed net distribution was commenced.
PHMC maintained entomological surveillance at five routine ‘sentinel’ sites representative of key locations in lowland Mimika from 1996 onwards. At each site, human-landing collections (HLCs) of mosquitoes were conducted at least once per week by 2 to 5 trained collectors for 5 to 10 hours during evening hours. Captured mosquitoes were examined and identified by microscopy and their species recorded based on key morphological characters. Data were combined to derive a human biting index over time. Automated daily rainfall was recorded at Kuala Kencana township, representing one of the 12 subdistricts in Mimika. Routine entomology and meteorological data were available from January 2004 until June 2009.
Data preparation and statistical analysis
The analysis was conducted according to an a priori statistical plan (S1 Text) and is reported according to RECORD (S1 RECORD Checklist). Additional multivariable regression analyses, requested at statistical review, were also undertaken of key outcomes controlling for population size, vector biting, and monthly time trends. Data from the hospitals, community clinics, and meteorological and entomological surveillance were aggregated by calendar month. The surveillance period was divided into four periods: pre–policy change (April 2004 to March 2006), early transition (April 2006 to March 2008, an equal interval to that observed before policy change), late transition (April 2008 to December 2009, corresponding to the end of the community and entomology surveillance), and post transition (January 2010 to December 2013, corresponding to the period between the opening of the new RSUD hospital and the end of the study period) (S1 Fig, S1 Table).
Estimated malaria incidence rates were derived for the pre-policy and the early and late transition periods using absolute numbers of malaria cases from both the hospital and community surveillance, the estimated population at the time (assuming linear growth between the censuses), and estimates of the proportion of febrile patients seeking treatment within the malariometric surveillance system, obtained from the two household surveys in 2005 and 2013 (S1 Fig). In a sensitivity analysis, the incidence of malaria was also derived assuming no change in treatment-seeking behaviour. Analyses of malaria-related morbidity and mortality were limited to RSMM data, as the necessary information was not collected from the other sites. To account for monthly time trends, vector biting, and population growth, Poisson regression analyses of the data pre-2009 were performed to estimate the adjusted incidence rate ratios (IRRs) for falciparum and for vivax malaria for the late transition period versus the pre–policy change period. Similar analyses were undertaken for hospital admissions using binomial regression to estimate the risk ratio.
All graphing and statistical analysis was done in STATA version 15.1 (StataCorp, College Station, TX, United States). Temporal trends in outcomes were presented graphically over the entire study period. Comparisons of outcomes before and after policy change were made using medians (with interquartile ranges [IQRs]), proportions (n/N with absolute differences and binomial 95% confidence intervals [CIs]), incidence rates (per 1,000 person-years [py] with IRRs and Poisson 95% CIs). Comparisons of prospectively collected community and hospital surveillance data were restricted to the pre-policy and late transition periods to ensure inclusion of the community surveillance (which ended in December 2009) and to avoid bias associated with the opening of RSUD (which began admitting significant numbers of malaria patients in January 2010). p-Values were not presented, since numbers of cases from the community clinics and hospitals were large and statistical significance was achieved even in the absence of clinical significance.
Ethical approval
Ethical approval for this study was obtained from the Health Research Ethics Committees of the University of Gadjah Mada, Indonesia (KE/FK/544/EC), and Menzies School of Health Research, Darwin, Australia (HREC 10.1397).
Results
Rainfall and entomology
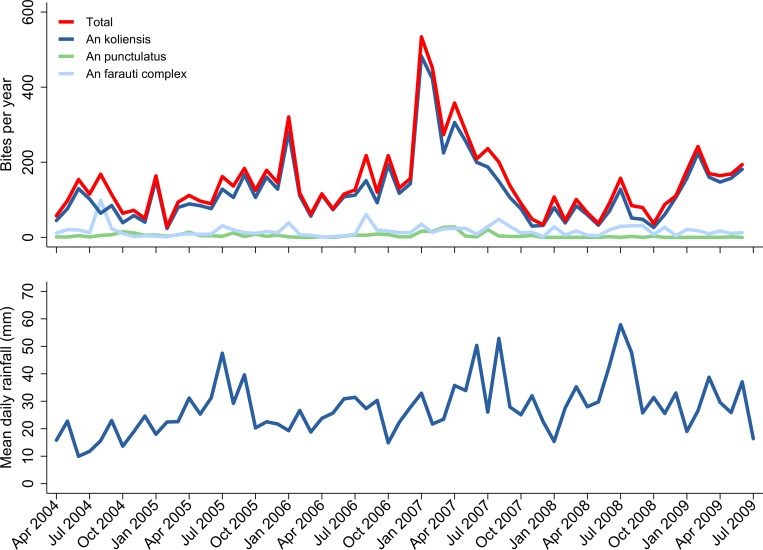
Between April 2004 and June 2009, the mean daily rainfall was 27.8 mm with small peaks in mid-2005, mid-2007, and mid-2008 (Fig 2, S1 Data). Over the same period, 204,968 human-hours of nighttime mosquito collections were conducted; the estimated mean number of anopheline bites per py was 145 (range 28 to 534) (Fig 2, S1 Data). There was a large peak in the number of mosquitoes caught throughout much of 2007; the mean estimated number of bites per py during this period was 238 (range 34 to 534). Overall, A. koliensis accounted for 85.0% (6,992/8,222) of mosquitoes captured by HLC, compared to 11.6% (956/8,222) for A. farauti species complex and 3.3% (274/8,222) for A. punctulatus.
Fig 2. Community entomological and meteorological surveillance.
Predicted number of Anopheles (‘An’) mosquito bites per year by month for the three main Plasmodium vectors in Mimika District (top) and mean daily rainfall by month (bottom).
Community facilities
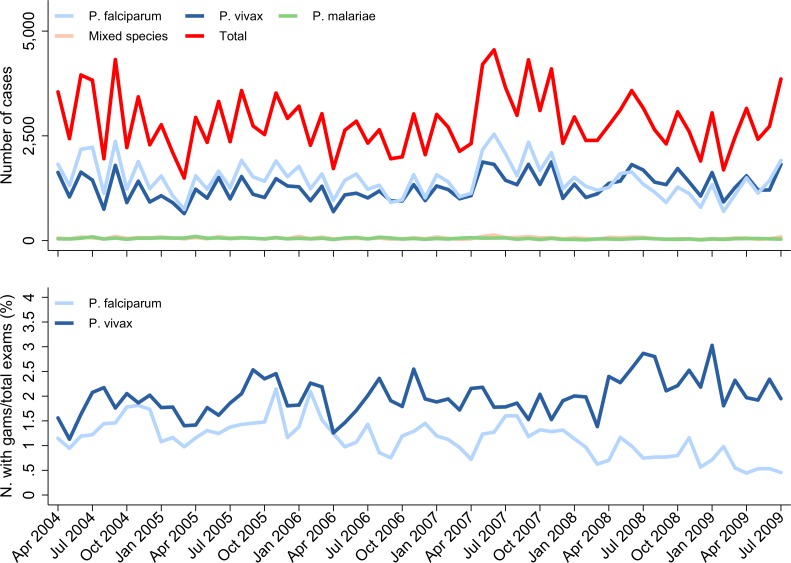
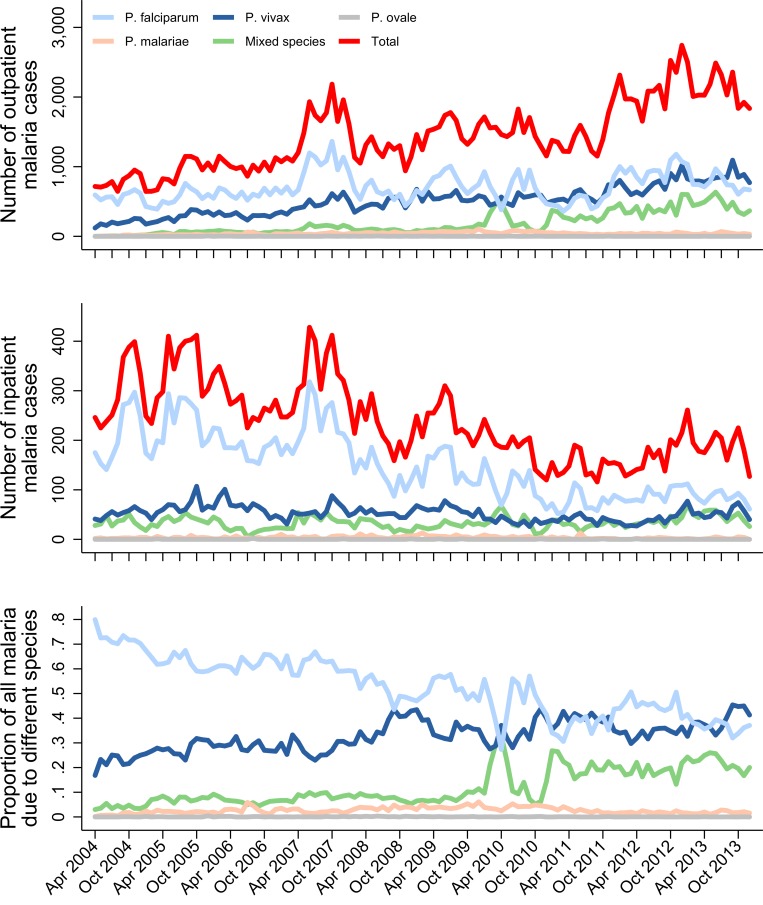
Data were gathered from 12 community outpatient facilities over a period of 69 months (April 2004 to December 2009). A total of 671,386 blood films were examined, of which 193,566 (28.8%) were positive for malaria; 98,530 (50.9%) were due to P. falciparum, 87,632 (45.3%) were due to P. vivax, 3,308 (1.7%) were due to P. malariae, and 4,096 (2.1%) were mixed species infections (Table 1). Slide positivity for P. falciparum declined from 14.8% (37,304/251,286) before treatment policy change to 13.0% (25,362/194,792) in the late transition period, a difference of −1.8% (95% CI −2.0% to −1.6%). Over the same period, the slide positivity for P. vivax increased from 11.5% (28,872/251,286) to 14.8% (28,861/194,792), difference = 3.3% (95% CI 3.1%–3.5%). The corresponding proportion of malaria infections due to P. vivax rose from 44.1% (30,444/69,098) to 53.3% (29,934/56,125), difference = 9.2% (95% CI 8.7%–9.8%) (Fig 3, S2 Data). In multivariable analyses controlling for population size, vector biting, and monthly time trends, the IRR for the late transition period compared with the pre–policy change period was 0.70 (95% CI 0.67–0.74) for falciparum malaria and 1.02 (95% CI 0.97–1.07) for vivax malaria.
Table 1. Patients presenting and diagnosed with malaria at community clinics and the RSMM hospital.
| Pre–Policy Change Period | Early Transition Period | Late Transition Period | Posttransition Period | Total | |
|---|---|---|---|---|---|
| Apr 2004–Mar 2006 | Apr 2006–Mar 2008 | Apr 2008–Dec 2009 | Jan 2010–Dec 2013 | ||
| Community Surveillance | |||||
| Total slide examinations | 251,286 | 225,308 | 194,792 | - | 671,386 |
| P. falciparum | 37,304 (14.8%) | 35,864 (15.9%) | 25,362 (13.0%) | - | 98,530 |
| P. vivax | 28,872 (11.5%) | 29,899 (13.3%) | 28,861 (14.8%) | - | 87,632 |
| P. malariae | 1,350 (0.5%) | 1,129 (0.5%) | 829 (0.4%) | - | 3,308 |
| Mixed species | 1,572 (0.6%) | 1,451 (0.6%) | 1,073 (0.6%) | - | 4,096 |
| Total malaria cases | 69,098 (27.5%) | 68,343 (30.3%) | 56,125 (28.8%) | - | 193,566 |
| P. falciparum gametocytes | 3,491 (1.4%) | 2,636 (1.2%) | 1,419 (0.7%) | - | 7,546 |
| P. vivax gametocytes | 4,700 (1.9%) | 4,192 (1.9%) | 4,403 (2.3%) | - | 13,295 |
| Outpatient presentations to the RSMM Hospital | |||||
| Total outpatient presentations | 155,029 | 181,493 | 169,391 | 453,506 | 959,419 |
| P. falciparum | 13,654 (8.8%) | 18,977 (10.5%) | 14,801 (8.7%) | 35,259 (7.8%) | 82,691 |
| P. vivax | 6,056 (3.9%) | 9,472 (5.2%) | 11,198 (6.6%) | 32,464 (7.2%) | 59,190 |
| P. malariae | 411 (0.3%) | 871 (0.5%) | 1,230 (0.7%) | 2,153 (0.5%) | 4,665 |
| P. ovale | 17 (0.01%) | 25 (0.01%) | 36 (0.02%) | 32 (0.01%) | 110 |
| Mixed species | 906 (0.6%) | 2,248 (1.2%) | 2,066 (1.2%) | 16,614 (3.7%) | 21,834 |
| All malaria | 21,044 (13.6%) | 31,593 (17.4%) | 29,331 (17.3%) | 86,522 (19.1%) | 168,490 |
| Hospital admissions at the RSMM Hospital | |||||
| Total hospital admissions | 19,260 | 20,293 | 16,921 | 38,781 | 95,255 |
| P. falciparum | 5,311 (27.6%) | 4,863 (24%) | 2,890 (17.1%) | 4,323 (11.1%) | 17,387 |
| P. vivax | 1,489 (7.7%) | 1,383 (6.8%) | 1,199 (7.1%) | 2,045 (5.3%) | 6,116 |
| P. malariae | 70 (0.4%) | 105 (0.5%) | 122 (0.7%) | 135 (0.3%) | 432 |
| P. ovale | 1 | 3 | 3 | 3 | 10 |
| Mixed species | 874 (4.5%) | 720 (3.5%) | 572 (3.4%) | 1,779 (4.6%) | 3,945 |
| All malaria | 7,745 (40.2%) | 7,074 (34.9%) | 4,786 (28.3%) | 8,285 (21.4%) | 27,890 |
Abbreviation: RSMM, Rumah Sakit Mitra Masyarakat.
Fig 3. Malaria surveillance gathered from community healthcare facilities.
Absolute number (‘N.’) of malaria cases detected within the community surveillance network by Plasmodium species and month (top) and proportion of all blood films examined in the community with gametocytes (‘gams’) by Plasmodium species and time (bottom).
The proportion of all blood films read that were positive for P. falciparum gametocytes fell steadily over the study period from 1.4% (3,491/251,286) pre–policy change to 0.7% (1,419/194,792) in the late transition period, a difference of −0.7% (95% CI −0.7% to −0.6%) (Fig 3). Over the same time interval, there was an increase in the overall gametocyte slide positivity for P. vivax, which rose from 1.9% (4,700/251,286) to 2.3% (4,403/194,792), a difference of 0.4% (95% CI 0.3%–0.5%).
RSMM hospital data
Data from RSMM were available for 117 months (April 2004 to December 2013). Overall, there were 1,054,674 patient presentations to the hospital, of which 196,380 (18.6%) were associated with malaria, 100,078 (51.0%) with P. falciparum, 65,306 (33.3%) with P. vivax, 5,097 (2.6%) with P. malariae, 120 (0.06%) with P. ovale, and 25,779 (13.1%) with mixed species infections. In total, 27,890 (14.2%) of the patients with malaria required admission to hospital, and 595 (0.3%) died in hospital. In the posttransition period, 22.5% (27,480/122,232) of malaria diagnosed at a tertiary facility was at the newly opened RSUD hospital (S3 Data); therefore, before-and-after comparisons at the RSMM hospital were only made between the pre–policy change era and the late transition period.
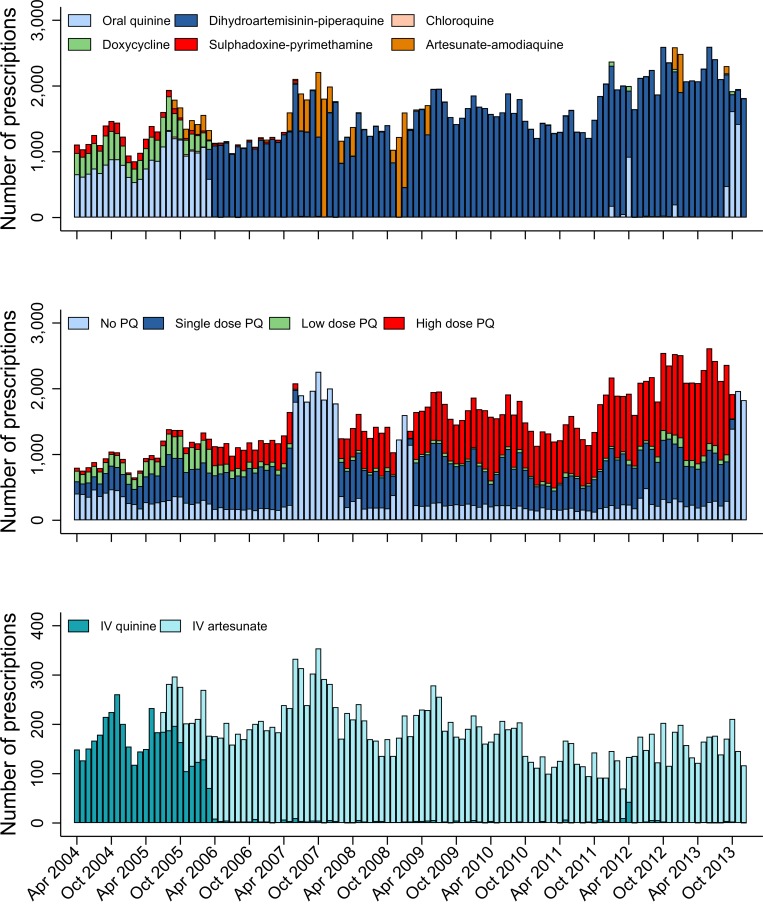
Before policy change, the most commonly prescribed blood schizontocide at RSMM hospital was oral quinine (20,364/24,538; 83.0%) (Fig 4); thereafter, DP was prescribed in 88.6% (139,002/156,902) and artesunate-amodiaquine in 6.0% (9,442/156,902) of malaria cases. Patients requiring IV therapy were prescribed quinine in 83.1% (3,858/4,641) of cases before policy change, but thereafter, 98.5% (16,414/16,661) were treated with IV artesunate. Before policy change, 63.5% (4,455/7,019) of patients with P. vivax malaria were treated with a 14-day primaquine regimen, and after policy change, this rose to 71.1% (51,344/72,196).
Fig 4. Antimalarial drug prescriptions at Mitra Masyarakat hospital.
Number of oral blood schizontocidal drug prescriptions (top), PQ prescriptions (middle), and IV blood schizontocidal drug prescriptions (bottom) at Mitra Masyarakat Hospital by month. Low-dose PQ is defined as a total dose of ≥1.5 to <5 mg/kg. High-dose PQ is defined as a total dose of ≥5 mg/kg. IV, intravenous; PQ, primaquine.
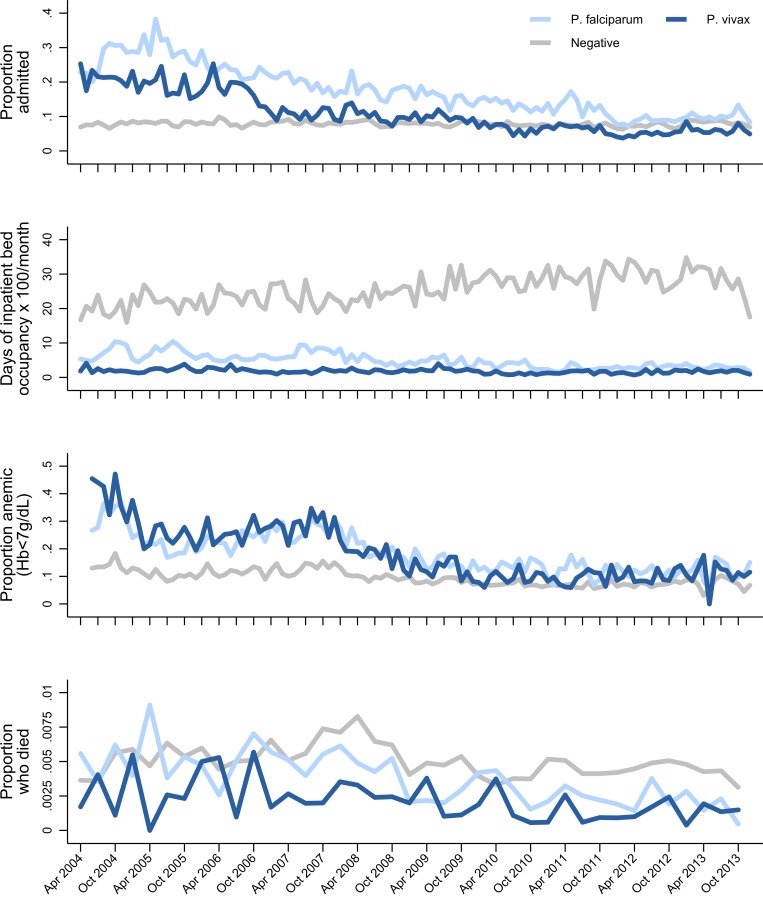
The proportion of all presentations to RSMM that were related to malaria rose from 16.5% (28,789/174,289) before policy change to 18.3% (34,117/186,312) in the late transition period (difference of 1.8% [95% CI 1.5%–2.0%]) (Fig 5). This was driven by a rise in the proportion of outpatients with malaria, which increased from 13.6% (21,044/155,029) to 17.3% (29,331/169,391), a difference of 3.7% (95% CI 3.5%–4.0%). Over the same period, the proportion of inpatients with malaria fell from 40.2% (7,745/19,260) to 28.3% (4,786/16,921; difference of −11.9% [95% CI −12.9% to −11.0%]), and the proportion of patients with malaria requiring admission fell from 26.9% (7,745/28,789) to 14.0% (4,786/34,117; difference of −12.9% [95% CI −13.5% to −12.2%]) (Fig 6, Table 1 and S3 Data). In multivariable analyses comparing the pre–policy change and late transition period, after controlling for monthly trends, the proportion of inpatients with malaria decreased by 0.56-fold (95% CI 0.51–0.60), and the proportion of patients with malaria requiring admission fell by 0.82-fold (95% CI 0.75–0.90).
Fig 5. Malaria cases at Mitra Masyarakat hospital.
Number of outpatient (top) and inpatient (middle) malaria cases at Mitra Masyarakat Hospital by Plasmodium species and month and proportion of all hospital malaria cases due to the different Plasmodium species (bottom).
Fig 6. Malaria-related morbidity and mortality at Mitra Masyarakat hospital.
Proportion of all patients presenting to Mitra Masyarakat Hospital who were admitted by Plasmodium species and month (top). Total number of days of inpatient bed occupancy × 100/month by Plasmodium species (second from top). Proportion of all patients presenting to Mitra Masyarakat Hospital who were anaemic (Hb < 7 g/dL) (third from top) and who died (bottom) by Plasmodium species and month. Hb, haemoglobin.
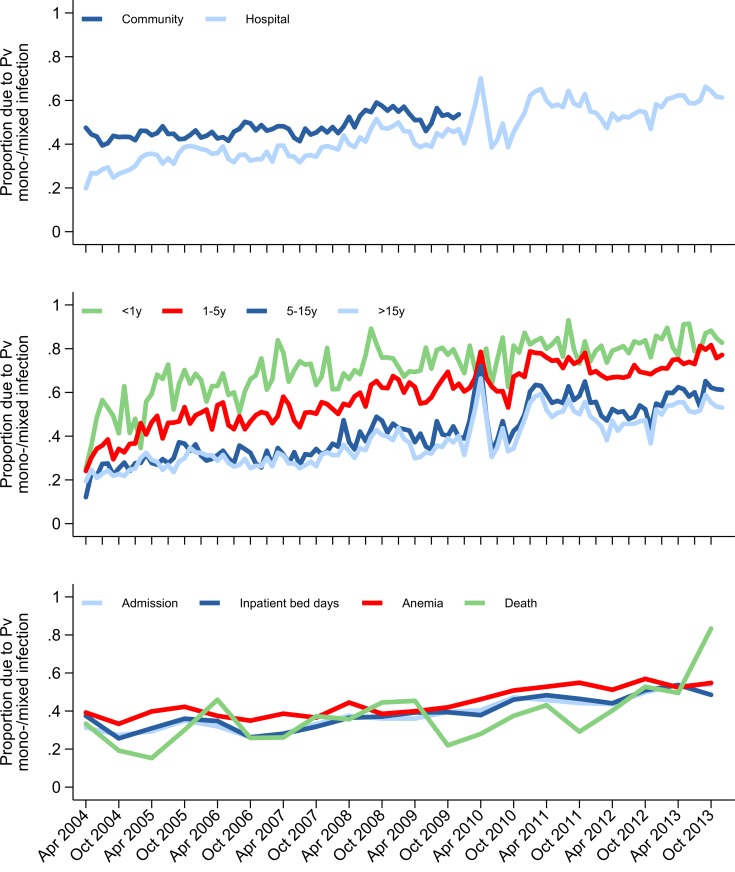
Of the patients admitted with malaria, the median length of stay decreased from 3 days (IQR 2–4) to 2 days (IQR 2–4), and this was associated with a fall in the median total monthly inpatient bed occupancy due to malaria from 1,033.5 days (IQR 897–1,251.5) pre–policy change to 769.5 days (IQR 685.5–856) post policy change (Fig 6). Overall, the proportion of malaria cases due to P. vivax mono- or mixed species infection rose from 32.4% (9,325/28,789) to 44.1% (15,035/34,117), a difference of 11.7% (95% CI 10.9%–12.4%) (Fig 7). Malaria due to any Plasmodium species collectively accounted for 57.7% (4,388/7,603) of all severe anaemia at the hospital before policy change and 41.7% (1,928/4,627) in the late transition period, a difference of −16.0% (95% CI −17.8% to −14.2%) (Fig 7).
Fig 7. Proportion of malaria morbidity and mortality attributable to Pv mono- or mixed infection.
In the community and hospital (top). In the hospital stratified by age group (middle). In the hospital: malaria-related hospital admissions, inpatient bed days, malaria-related anaemia, and malaria-related deaths (bottom). Pv, P. vivax.
Malaria-attributable mortality
Before policy change, malaria accounted for 15.5% (137/886) of all deaths at RSMM, with 0.48% (137/28,789) of patients who presented with malaria dying during their hospital encounter. In the late transition period, the corresponding figures were 10.4% (100/961; difference = −5.1% [95% CI −8.1 to −2.0]) and 0.29% (100/34,117; difference = −0.18% [95% CI −0.28 to −0.08]) (Fig 6, S3 Data). Over the same period, there was a significant fall in the mortality attributable to P. falciparum (0.53% [100/18,965] versus 0.32% [57/17,691]; difference = −0.21% [95% CI −0.34 to −0.07]), but this was not apparent for P. vivax (0.28% [21/7,545] versus 0.23% [28/12,397]; difference = −0.05% [95% CI −0.20 to 0.09]) or nonmalarial disease (0.51% [749/145,500] versus 0.57% [861/152,195], difference = 0.05% [95% CI −0.002 to 0.10]). Overall, P. vivax accounted for 15.3% (21/137) of malaria-related deaths pre–policy change and 28.0% (28/100) in the late transition period, a difference of 12.7% (95% CI 2.0%–23.3%) (S3 Data).
Assuming that 30% of deaths in the region occurred at RSMM hospital, the overall malaria-attributable mortality rate in the population of Mimika District fell from 1.90 (95% CI 1.73–2.08) per thousand py before the policy change to 1.29 (95% CI 1.15–1.43) per 1,000 py in the late transition period, a rate difference of −0.61 (95% CI −0.83 to −0.39) per 1,000 py.
Combined estimates of malaria incidence
When community and hospital surveillance data were combined, a total of 295,971 cases of malaria were diagnosed between April 2004 and December 2009. The overall incidence of malaria was 406 (95% CI 404–409) per 1,000 py before policy change, 372 (95% CI 370–374) per 1,000 py in the early transition period, and 351 (95% CI 349–354) per 1,000 py in the late transition period (Table 2, S4 Data). Assuming that 45.7% of patients with malaria were detected by the surveillance network pre–policy change and 67.3% in the late and posttransition periods, the overall incidence of P. falciparum fell from 511 to 249 per 1,000 py (IRR = 0.49 [95% CI 0.48–0.49]), whereas the incidence of P. vivax fell from 331 to 239 per 1,000 py (IRR = 0.72 [95% CI 0.71–0.73]). In a sensitivity analysis assuming no shift in treatment-seeking behaviour, the incidence of P. falciparum fell from 234 to 168 per 1,000 py (IRR = 0.72 [95% CI 0.71–0.73]), whereas the incidence of P. vivax rose from 152 to 161 per 1,000 py (IRR = 1.06 [95% CI 1.05–1.08]).
Table 2. Total number of community and hospital malaria cases and incidence of malaria before and after policy change.
| Pre–Policy Change Period | Early Transition Period | Late Transition Period | Posttransition Period | |
|---|---|---|---|---|
| Apr 2004–Mar 2006 | Apr 2006–Mar 2008 | Apr 2008–Dec 2009 | Jan 2010–Dec 2013 | |
| Population of Mimikaa | 120,457 | 143,723 | 148,124 | 189,447 |
| Months of observation | 24 | 24 | 21 | 48 |
| Person-years of observation | 240,914 | 287,446 | 259,217 | 757,788 |
| Percent of patients with malaria attending the surveillance networkb | 45.7% | - | - | 67.3% |
| Number of malaria cases | ||||
| P. falciparum | 56,269 | 59,704 | 43,464 | 53,749c |
| P. vivax | 36,417 | 40,754 | 41,659 | 46,491c |
| P. malariae | 1,831 | 2,105 | 2,193 | 2,477c |
| Mixed species | 3,352 | 4,419 | 3,719 | 19,515c |
| Overalld | 97,887 | 107,010 | 91,074 | 122,267c |
| Incidence rate of malaria (per 1,000 person-years) assuming complete ascertainment of all malaria patients (95% confidence interval) | ||||
| P. falciparum | 234 (232–236) | 208 (206–209) | 168 (166–169) | - |
| P. vivax | 152 (150–153) | 142 (140–143) | 161 (159–162) | - |
| P. malariae | 7.6 (7.3–8.0) | 7.3 (7.0–7.6) | 8.5 (8.1–8.8) | - |
| Mixed species | 13.9 (13.4–14.4) | 15.4 (14.9–15.8) | 14.3 (13.9–14.8) | - |
| Overall | 406 (404–409) | 372 (370–375) | 351 (349–354) | - |
| Incidence rate of malaria (per 1,000 person-years) assuming shifts in treatment-seeking behaviour (95% confidence interval) | ||||
| P. falciparum | 511 (508–514) | - | 249 (247–251) | - |
| P. vivax | 331 (328–333) | - | 239 (237–241) | - |
| P. malariae | 16.6 (16.1–17.2) | - | 12.6 (12.1–13.0) | - |
| Mixed species | 30.4 (29.8–31.2) | - | 21.3 (20.8–21.9) | - |
| Overall | 889 (885–893) | - | 522 (519–525) | - |
aPopulation estimates taken at midpoint of the time interval.
bEstimates for the proportion of patients with malaria attending the public provider and thus who will have been detected by the surveillance network were derived from household surveys conducted in 2005 and 2013, reported previously [28].
cIncludes patients attending the new RSUD hospital but excludes community cases due to surveillance finishing in December 2009.
dIncludes 120 cases of P. ovale.
Abbreviation: RSUD, Rumah Sakit Umum Daerah.
Discussion
In March 2006, Indonesia was the first malaria-endemic country to adopt a unified schizontocidal treatment policy for malaria due to any Plasmodium species: DP for uncomplicated malaria and IV artesunate for severe malaria. These changes came on a background of failing treatment regimens due to high-grade multidrug resistance in both P. falciparum and P. vivax species. In Mimika District, southern Papua, the uptake of the new policy was rapid, with the new treatment regimens adopted into practice in most public health facilities within a month. In this high-transmission setting, we found that the implementation of highly effective antimalarial treatment regimens was associated with a marked reduction in both malaria-related morbidity and mortality. The incidence of malaria and the proportion of malaria requiring admission to hospital fell by one-half, bed occupancy of patients with malaria fell by 26%, and malaria-related mortality fell by one-third. Associated with these changes, the proportion of patients with malaria attributable to P. vivax increased from 41% to 54%, and the proportion of malaria-related deaths attributable to P. vivax rose from 15% to 28%.
Our study highlights the complexity of defining temporal changes in the burden of malaria at a population level over a long period of time [1,2,29]. During the 9 years of surveillance, there was a substantial increase in the local population, fluctuations in rainfall and vector mosquito numbers, and marked changes in healthcare provision and treatment-seeking behaviour. Our comprehensive surveillance quantified patient numbers at the only lowland hospital (until late 2009) and all public and mine-supported clinics, which collectively diagnosed and treated almost half a million cases of malaria over the study period. Although these clinics offered healthcare for free or at a nominal cost, our treatment-seeking surveys suggested that initially only 46% of patients with malaria sought treatment in the public sector. However, following the change in policy, there was a significant shift in behaviour, with 67% of patients seeking treatment in the public sector where they could access DP, a drug perceived to be highly effective compared to previously available treatments [28]. This shift in behaviour, along with the rise in the total population and an increase in vector numbers in 2007 (Fig 2), may have contributed to the initial surge in malaria cases and slide positivity observed in the community and hospital outpatients department in the early transition period but a subsequent fall in these metrics in the late transition period as the impact of ACT on P. falciparum began to manifest (Fig 3). Conversely, in the latter part of the study, a new public hospital facility (RSUD) outside of the initial surveillance network was opened and assumed care of approximately 20% of malaria inpatients, acting to artificially decrease the burden of malaria at RSMM hospital. To control for these confounding factors, the overall temporal trends by month are presented, but the comparisons pre–and post–policy change were restricted conservatively to the period immediately before policy change and the late transition period, prior to the opening of the new hospital facility.
The replacement of failing treatment regimens with highly efficacious schizontocidal treatment has potential to reduce malaria-related morbidity and mortality and decrease the risk of recurrent parasitaemia and ongoing transmission [30]. In Papua in 2005, the efficacy of DP against P. falciparum and P. vivax was greater than 95%, with antimalarial efficacy sustained against both species throughout the study period [26,31]. In the same year, a clinical trial of patients with severe malaria at the RSMM hospital demonstrated that IV artesunate reduced associated mortality by 35% compared to IV quinine [27]. Our analysis highlights that, following implementation of both of these artemisinin-based treatment strategies in April 2006, there was a significant reduction in malaria-related morbidity and mortality, which was most apparent at the RSMM hospital. Whilst outpatient numbers actually increased over the study period, both the absolute number of malaria admissions and the proportion of malaria patients requiring admission fell (the latter from 27% to 14%). This was associated with a marked reduction in total bed occupancy due to malaria, shorter admission times, and a lower risk of severe anaemia- and malaria-related mortality. In total, 264 bed days per month were made available at the hospital for the treatment of other diseases. These findings are consistent with African studies that have shown that the most prominent impact of enhanced malaria control activities is a reduction in severe malaria and mortality [32,33]. The variation in malaria morbidity was less marked in the community, where absolute numbers of patients remained high and where there was only a modest fall in malaria prevalence from 16.3% to 12.2%. However, after accounting for population growth and shifts in treatment-seeking behaviour, the estimated overall incidence of malaria in the community also fell significantly.
A consistent finding in both the hospital and community setting was the marked increase in the proportion of malaria caused by P. vivax. Policy change was associated with a differential variation between species in the overall cases of malaria, severe disease, and gametocyte carriage. At the start of the study, P. vivax accounted for 32% of all malaria at the hospital and 44% in the community. By the late transition period, P. vivax was the predominant cause of malaria, accounting for 54% of all malaria cases. Whereas the overall risk of mortality in patients presenting with P. falciparum fell from 0.53% to less than 0.25% in the posttransition period, there was no fall in mortality associated with P. vivax, possibly reflecting a higher likelihood of concomitant nonmalarial morbidities in severely ill patients with P. vivax malaria [34,35]. In the community, the proportion of patients with P. falciparum gametocytes on blood film examination halved (from 1.4% to 0.7%), whereas the proportion of patients with P. vivax gametocytes remained unchanged at about 2% (Fig 3).
P. vivax is less amenable than P. falciparum to control by enhanced or scaled-up antimalarial treatment efforts [6,7,36]. Mass drug administration that does not include antirelapse therapy has little effect on P. vivax [3]. There are several biological reasons for this refractoriness. Firstly, P. vivax gametocytes appear early during the course of an infection and are therefore more likely than P. falciparum gametocytes, which appear later, to have been transmitted to mosquitoes prior to antimalarial treatment. Secondly, failure to sterilize the liver of hypnozoites can result in multiple subsequent relapses and thus much greater transmission potential from a single inoculation of P. vivax as compared with P. falciparum. Thirdly, immunity to P. vivax develops early in endemic regions because of the high force of infection from relapses [22,37]. This results in a large pool of asymptomatic patients who probably still harbour gametocytes and therefore remain infectious to mosquitoes [38,39].
Although DP provides posttreatment prophylaxis against P. vivax recurrence for up to 42 days, it has minimal effect on P. vivax relapses thereafter. At the same time as the change in blood schizontocidal treatment policy, the recommended dose of primaquine for radical cure of P. vivax infections was revised from a total dose of 3.5 mg/kg to 7 mg/kg to treat the relatively primaquine-tolerant P. vivax strains in Papua [40]. If safely and effectively delivered, high-dose primaquine regimens should produce a significant reduction in P. vivax transmission. However, in Timika, the provision of unsupervised high-dose primaquine combined with DP has, at best, a modest effect on the likelihood of representation to hospital with vivax malaria [16]. We postulate that nonadherence to unsupervised primaquine is one of the most likely explanations for the minimal decline in P. vivax incidence in the region.
Our study has several important strengths. The multifaceted surveillance system incorporated prospectively collected community and hospital data along with before-and-after cross-sectional surveys, providing a means of checking the consistency of the findings across various settings and thus increasing confidence in the internal validity of our results. Data on nonmalaria presentations to hospital enabled us to control the hospital data for population growth by presenting the proportional burden of malaria over time. High-quality microscopy services both at the hospital and in the community clinics are maintained by accredited microscopists, whose performance is reviewed regularly [22]. Consistent procedures for examination and reporting of blood films therefore support the validity of longitudinal assessments of Plasmodium species distributions observed in our analysis. Individualized clinical data at RSMM with linkage to pharmacy and haematology data enabled a very large-scale assessment of the temporal trends in malaria-related morbidity (in addition to incidence) in the hospital population.
Our study also has some significant limitations. Estimates of the local population were imprecise because of large transient migrant groups who were excluded from censuses. This was an issue particularly in the latter years, when the population at risk of malaria may have been underestimated; thus, our estimates of the reduction in malaria incidence are likely to be conservative. The estimated change in the proportion of malaria patients detected by the surveillance network was derived from two assessments of treatment-seeking behaviour in 2005 and 2013 [28]. In a sensitivity analysis, assuming no shift in treatment-seeking behaviour, the estimated reduction in the incidence of falciparum malaria was only 28%, with a 6% rise in the incidence P. vivax malaria. However, changes in treatment-seeking behaviour should not have confounded estimates of the proportion of malaria due to P. vivax or the reduction in hospital-related morbidity. The surveillance system captured changes in human–mosquito attack rates and climate variability but did not record concurrent vector-control interventions. Throughout most of the study period, formal vector-control activities were supported by the mine-supported PHMC program. These activities remained relatively constant, apart from a large bed net distribution and IRS program in urban Timika that commenced in 2013, towards the end of the posttransition period. Changes in vector-control measures were therefore unlikely to have influenced to a significant extent the reduction in malaria incidence between the pre–policy change and late transition periods. Finally, in view of the variations in treatment-seeking behaviour and healthcare provision, an a priori decision was made to compare the malarial outcomes between the pre-policy and the late transition periods. Although comparison of dichotomised temporal data can result in loss of power and in type I error, multivariable regression analyses of unconstrained monthly data confirmed the observed trends and demonstrated that they were still apparent after controlling for population growth and vector biting.
In summary, in Papua, Indonesia, a change in antimalarial treatment policy from failing drugs to highly efficacious artemisinin-based treatment for both uncomplicated and severe malaria was associated with a modest reduction in the overall incidence of malaria but a significant reduction in malaria-related hospital admissions and mortality. In this area coendemic for both P. falciparum and P. vivax, there was a marked increase in the proportion of malaria attributable to P. vivax. Additional scale-up of the existing treatment strategy is likely to result in further reductions in P. falciparum transmission; however, in order to reduce P. vivax transmission significantly, antirelapse therapy will need to be delivered more effectively. Novel strategies are being developed to improve primaquine adherence through community education campaigns and directly observed supervision of treatment [41]. The availability of point-of-care testing for G6PD deficiency raises the possibility of introducing short-course high-daily-dose primaquine regimens and tafenoquine that will facilitate further adherence to a complete course of treatment [42,43]. In coendemic regions, access to safe and effective radical cure of malaria for all patients at risk of malaria will be critical for the timely elimination of malaria.
Supporting information
(DOCX)
(DOCX)
First and second household surveys were reported previously [28].
(TIF)
(DOCX)
(XLSX)
(XLSX)
RSMM, Rumah Sakit Mitra Masyarakat.
(XLSX)
RSMM, Rumah Sakit Mitra Masyarakat; RSUD, Rumah Sakit Umum Daerah.
(XLSX)
Acknowledgments
We would like to thank the staff of Rumah Sakit Mitra Masyarakat. We also thank Emiliana Tjitra, Mohamed Karyana, Maurits Okoseray, Claude Faurant, Morrison Bethea, Peter Ebsworth, and Ram Vemuri for their support in setting up the study and Norman Price for his topographical assistance. We are grateful to Lembaga Pengembangan Masyarakat Amungme Kamoro, the Mimika District Health Office, and the PT Freeport Indonesia Public Health Malaria Control Department for their ongoing support.
Abbreviations
- ACT
artemisinin combination therapy
- CI
confidence interval
- DP
dihydroartemisinin plus piperaquine
- G6PD
glucose-6-phosphate dehydrogenase
- HLC
human-landing collection
- IQR
interquartile range
- IRR
incidence rate ratio
- IRS
indoor residual spraying
- IV
intravenous
- LLIN
long-lasting insecticide-treated bed net
- PHMC
Public Health Malaria Control
- py
person-years
- RSMM
Rumah Sakit Mitra Masyarakat
- RSUD
Rumah Sakit Umum Daerah
Data Availability
The relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was funded by the Wellcome Trust (Senior Fellowship in Clinical Science to RNP 200909), the Bill and Melinda Gates Foundation (OPRA, OPP1054404), and the Australian National Health and Medical Research Council (the HOT NORTH initiative #1131932 and Fellowships to JAS #110975 and NMA #1135820; and the Australian Centre of Research Excellence on Malaria Elimination, ACREME #1134989). The Timika Research Facility and Papuan Community Health Foundation is supported by the Australian Department of Foreign Affairs and Trade (DFAT #74431). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Barnes KI, Durrheim DN, Little F, Jackson A, Mehta U, Allen E, et al. Effect of artemether-lumefantrine policy and improved vector control on malaria burden in KwaZulu-Natal, South Africa. PLoS Med. 20052(11):e330 Epub 2005/09/29. 10.1371/journal.pmed.0020330 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattarai A, Ali AS, Kachur SP, Martensson A, Abbas AK, Khatib R, et al. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 2007;4(11):e309 Epub 2007/11/09. 10.1371/journal.pmed.0040309 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landier J, Parker DM, Thu AM, Lwin KM, Delmas G, Nosten FH, et al. Effect of generalised access to early diagnosis and treatment and targeted mass drug administration on Plasmodium falciparum malaria in Eastern Myanmar: an observational study of a regional elimination programme. Lancet. 2018;391(10133):1916–26. Epub 2018/04/29. 10.1016/S0140-6736(18)30792-X . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO). World Malaria Report: 2014. Geneva: WHO; 2014. [Google Scholar]
- 5.Douglas NM, Simpson JA, Phyo AP, Siswantoro H, Hasugian AR, Kenangalem E, et al. Gametocyte Dynamics and the Role of Drugs in Reducing the Transmission Potential of Plasmodium vivax. J Infect Dis. 2013. 10.1093/infdis/jit261 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sattabongkot J, Tsuboi T, Zollner GE, Sirichaisinthop J, Cui L. Plasmodium vivax transmission: chances for control? Trends Parasitol. 2004;20(4):192–8. Epub 2004/04/22. 10.1016/j.pt.2004.02.001 . [DOI] [PubMed] [Google Scholar]
- 7.Oliveira-Ferreira J, Lacerda MV, Brasil P, Ladislau JL, Tauil PL, Daniel-Ribeiro CT. Malaria in Brazil: an overview. Malar J. 2010;9:115 Epub 2010/05/04. 10.1186/1475-2875-9-115 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abeyasinghe RR, Galappaththy GN, Smith Gueye C, Kahn JG, Feachem RG. Malaria control and elimination in Sri Lanka: documenting progress and success factors in a conflict setting. PLoS ONE. 2012;7(8):e43162 10.1371/journal.pone.0043162 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.John GK, Douglas NM, von Seidlein L, Nosten F, Baird JK, White NJ, et al. Primaquine radical cure of Plasmodium vivax: a critical review of the literature. Malar J. 2012;11:280 10.1186/1475-2875-11-280 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abreha T, Hwang J, Thriemer K, Tadesse Y, Girma S, Melaku Z, et al. Comparison of artemether-lumefantrine and chloroquine with and without primaquine for the treatment of Plasmodium vivax infection in Ethiopia: A randomized controlled trial. PLoS Med. 2017;14(5):e1002299 10.1371/journal.pmed.1002299 eCollection 2017 May. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelwan EJ, Ekawati LL, Tjahjono B, Setiabudy R, Sutanto I, Chand K, et al. Randomized trial of primaquine hypnozoitocidal efficacy when administered with artemisinin-combined blood schizontocides for radical cure of Plasmodium vivax in Indonesia. BMC Med. 2015;13(1):294 10.1186/s12916-015-0535-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thriemer K, Bobogare A, Ley B, Gudo CS, Alam MS, Anstey NM, et al. Quantifying primaquine effectiveness and improving adherence: a round table discussion of the APMEN Vivax Working Group. Malar J. 2018;17(1):241 Epub 2018/06/22. 10.1186/s12936-018-2380-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira EA, Ishikawa EA, Fontes CJ. Adherence to Plasmodium vivax malaria treatment in the Brazilian Amazon Region. Malar J. 2011;10:355 10.1186/1475-2875-10-355 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maneeboonyang W, Lawpoolsri S, Puangsa-Art S, Yimsamran S, Thanyavanich N, Wuthisen P, et al. Directly observed therapy with primaquine to reduce the recurrence rate of plasmodium vivax infection along the Thai-Myanmar border. Southeast Asian J Trop Med Public Health. 2011;42(1):9–18. Epub 2011/02/18. . [PubMed] [Google Scholar]
- 15.Khantikul N, Butraporn P, Kim HS, Leemingsawat S, Tempongko MA, Suwonkerd W. Adherence to antimalarial drug therapy among vivax malaria patients in northern Thailand. J Health Popul Nutr. 2009;27(1):4–13. Epub 2009/03/03. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douglas NM, Poespoprodjo JR, Patriani D, Malloy MJ, Kenangalem E, Sugiarto P, et al. Unsupervised primaquine for the treatment of Plasmodium vivax malaria relapses in southern Papua: A hospital-based cohort study. PLoS Med. 2017;14(8):e1002379 Epub 2017/08/30. 10.1371/journal.pmed.1002379 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karyana M, Burdarm L, Yeung S, Kenangalem E, Wariker N, Maristela R, et al. Malaria morbidity in Papua Indonesia, an area with multidrug resistant Plasmodium vivax and Plasmodium falciparum. Malar J. 2008;7:148 10.1186/1475-2875-7-148 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ratcliff A, Siswantoro H, Kenangalem E, Wuwung M, Brockman A, Edstein MD, et al. Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine-pyrimethamine in southern Papua, Indonesia. Trans R Soc Trop Med Hyg. 2007;101(4):351–9. 10.1016/j.trstmh.2006.06.008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell B, Chalfein F, Prasetyorini B, Kenangalem E, Piera K, Suwanarusk R, et al. Determinants of in vitro drug susceptibility testing of Plasmodium vivax. Antimicrob Agents Chemother. 2008;52(3):1040–5. 10.1128/AAC.01334-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumawinata IW, Bernadeta, Leksana B, Sutamihardja A, Purnomo, Subianto B, et al. Very high risk of therapeutic failure with chloroquine for uncomplicated Plasmodium falciparum and P. vivax malaria in Indonesian Papua. Am J Trop Med Hyg. 2003;68(4):416–20. . [PubMed] [Google Scholar]
- 21.Price RN, Douglas NM, Anstey NM. New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr Opin Infect Dis. 2009;22(5):430–5. Epub 2009/07/03. 10.1097/QCO.0b013e32832f14c1 . [DOI] [PubMed] [Google Scholar]
- 22.Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, Karyana M, et al. Multidrug-Resistant Plasmodium vivax Associated with Severe and Fatal Malaria: A Prospective Study in Papua, Indonesia. PLoS Med. 2008;5(6):e128 10.1371/journal.pmed.0050128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karyana M, Devine A, Kenangalem E, Burdarm L, Poespoprodjo JR, Vemuri R, et al. Treatment-seeking behaviour and associated costs for malaria in Papua, Indonesia. Malar J. 2016;15(1):536 Epub 2016/11/09. 10.1186/s12936-016-1588-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mimika District Health Office Annual Statistics. Timika, Papua, Indonesia: District Health Office; 2013. [Google Scholar]
- 25.Battle KE, Karhunen MS, Bhatt S, Gething PW, Howes RE, Golding N, et al. Geographical variation in Plasmodium vivax relapse. Malar J. 2014;13:144 10.1186/1475-2875-13-144 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratcliff A, Siswantoro H, Kenangalem E, Maristela R, Wuwung RM, Laihad F, et al. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet. 2007;369(9563):757–65. 10.1016/S0140-6736(07)60160-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dondorp A, Nosten F, Stepniewska K, Day N, White N, South East Asian Quinine Artesunate Malaria Trial group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366(9487):717–25. Epub 2005/08/30. 10.1016/S0140-6736(05)67176-0 . [DOI] [PubMed] [Google Scholar]
- 28.Devine A, Kenangalem E, Burdam FH, Anstey NM, Poespoprodjo JR, Price RN, et al. Treatment-Seeking Behavior after the Implementation of a Unified Policy of Dihydroartemisinin-Piperaquine for the Treatment of Uncomplicated Malaria in Papua, Indonesia. Am J Trop Med Hyg. 2018;98(2):543–50. Epub 2017/12/28. 10.4269/ajtmh.17-0680 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ceesay SJ, Casals-Pascual C, Erskine J, Anya SE, Duah NO, Fulford AJ, et al. Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. Lancet. 2008;372(9649):1545–54. 10.1016/S0140-6736(08)61654-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrara VI, Lwin KM, Phyo AP, Ashley E, Wiladphaingern J, Sriprawat K, et al. Malaria burden and artemisinin resistance in the mobile and migrant population on the thai-myanmar border, 1999–2011: an observational study. PLoS Med. 2013;10(3):e1001398 10.1371/journal.pmed.1001398 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poespoprodjo JR, Kenangalem E, Wafom J, Chandrawati F, Puspitasari AM, Ley B, et al. Therapeutic Response to Dihydroartemisinin-Piperaquine for P. falciparum and P. vivax Nine Years after Its Introduction in Southern Papua, Indonesia. Am J Trop Med Hyg. 2018;98(3):677–82. Epub 2018/01/19. 10.4269/ajtmh.17-0662 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nankabirwa JI, Briggs J, Rek J, Arinaitwe E, Nayebare P, Katrak S, et al. Persistent parasitemia despite dramatic reduction in malaria incidence after 3 rounds of indoor residual spraying in Tororo, Uganda. J Infect Dis. 2018. Epub 2018/11/02. 10.1093/infdis/jiy628 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Alessandro U, Olaleye BO, McGuire W, Langerock P, Bennett S, Aikins MK, et al. Mortality and morbidity from malaria in Gambian children after introduction of an impregnated bednet programme. Lancet. 1995;345(8948):479–83. Epub 1995/02/25. . [DOI] [PubMed] [Google Scholar]
- 34.Douglas NM, Pontororing GJ, Lampah DA, Yeo TW, Kenangalem E, Poespoprodjo JR, et al. Mortality attributable to Plasmodium vivax malaria: a clinical audit from Papua, Indonesia. BMC Med. 2014;12:217 10.1186/s12916-014-0217-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anstey NM, Douglas NM, Poespoprodjo JR, Price RN. Plasmodium vivax: Clinical Spectrum, Risk Factors and Pathogenesis. Adv Parasitol. 2012;80:151–201. 10.1016/B978-0-12-397900-1.00003-7 . [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez JC, Uribe GA, Araujo RM, Narvaez PC, Valencia SH. Epidemiology and control of malaria in Colombia. Mem Inst Oswaldo Cruz. 2011;106 Suppl 1:114–22. Epub 2011/09/09. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michon P, Cole-Tobian JL, Dabod E, Schoepflin S, Igu J, Susapu M, et al. The risk of malarial infections and disease in Papua New Guinean children. Am J Trop Med Hyg. 2007;76(6):997–1008. . [PMC free article] [PubMed] [Google Scholar]
- 38.Nguitragool W, Mueller I, Kumpitak C, Saeseu T, Bantuchai S, Yorsaeng R, et al. Very high carriage of gametocytes in asymptomatic low-density Plasmodium falciparum and P. vivax infections in western Thailand. Parasit Vectors. 2017;10(1):512 Epub 2017/10/27. 10.1186/s13071-017-2407-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman RE, Kumpitak C, Ponlawat A, Maneechai N, Phunkitchar V, Rachapaew N, et al. Infectivity of asymptomatic Plasmodium-infected human populations to Anopheles dirus mosquitoes in western Thailand. J Med Entomol. 2004;41(2):201–8. . [DOI] [PubMed] [Google Scholar]
- 40.Baird JK. Resistance to therapies for infection by Plasmodium vivax. Clin Microbiol Rev. 2009;22(3):508–34. 10.1128/CMR.00008-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thriemer K, Ley B, Bobogare A, Dysoley L, Alam MS, Pasaribu AP, et al. Challenges for achieving safe and effective radical cure of Plasmodium vivax: a round table discussion of the APMEN Vivax Working Group. Malar J. 2017;16(1):141 10.1186/s12936-017-1784-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lacerda MVG, Llanos-Cuentas A, Krudsood S, Lon C, Saunders DL, Mohammed R, et al. Single-Dose Tafenoquine to Prevent Relapse of Plasmodium vivax Malaria. N Engl J Med. 2019;380(3):215–28. Epub 2019/01/17. 10.1056/NEJMoa1710775 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.IMPROV Study Group. Improving the radical cure of vivax malaria (IMPROV): a study protocol for a multicentre randomised, placebo-controlled comparison of short and long course primaquine regimens. BMC Infect Dis. 2015;15:558 Epub 2015/12/09. 10.1186/s12879-015-1276-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
First and second household surveys were reported previously [28].
(TIF)
(DOCX)
(XLSX)
(XLSX)
RSMM, Rumah Sakit Mitra Masyarakat.
(XLSX)
RSMM, Rumah Sakit Mitra Masyarakat; RSUD, Rumah Sakit Umum Daerah.
(XLSX)
Data Availability Statement
The relevant data are within the paper and its Supporting Information files.