Figure 7. Stabilization of yigL mRNA by coaxial base pairing with SgrS in E. coli.
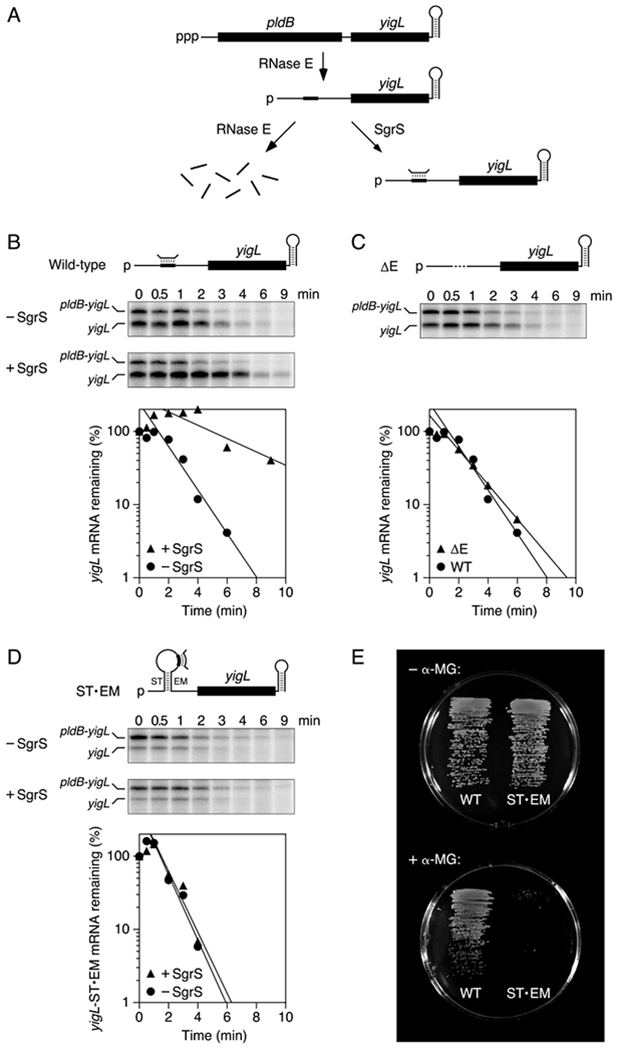
(A) SgrS-dependent pathway for the degradation of the dicistronic pldB-yigL transcript by RNase E in E. coli. Bent line, SgrS; broad line, SgrS-binding site; black rectangles, pldB and yigL translational units; ppp, 5′ triphosphate; p, 5′ monophosphate.
(B) Protection of wild-type yigL mRNA by SgrS in E. coli. Transcription in cells lacking or containing SgrS was arrested by treatment with rifampicin, and equal amounts of total cellular RNA isolated at time intervals thereafter were examined by S1 analysis. (Top) Diagram of the yigL 5′ UTR; (middle) gel images; (bottom) semilogarithmic graph of the amount of yigL mRNA remaining as a function of time. Representative experiments are shown.
(C) Rapid degradation of yigLΔE mRNA in E. coli cells lacking SgrS. Dashed line, deleted site of RNase E cleavage and SgrS binding. The graph compares the decay rates of yigLΔE and wild-type yigL in the absence of SgrS. A representative experiment is shown.
(D) Inability of SgrS to protect yigL-ST•EM mRNA from rapid degradation in E. coli. ST and EM, complementary RNA segments inserted upstream and downstream of the SgrS binding site. Representative experiments are shown. See also Figure S7.
(E) Importance of coaxial base pairing with the yigL 5′ UTR for the ability of SgrS to protect E. coli from α-methylglucoside toxicity. Isogenic E. coli cells whose chromosome contained either a wild-type yigL allele (left) or a yigL-ST•EM allele (right) and in which SgrS was expressed from its native promoter were grown on minimal glycerol medium lacking (top) or containing (bottom) α-methylglucoside (α-MG). To avoid masking the effect of differences in yigL Mrna stability on YigL production and α-methylglucoside resistance, synthesis of the PtsG glucose transporter was rendered insensitive to repression by SgrS in both of these strains.
