Abstract
Substantial knowledge of auditory processing within mammalian nervous systems emerged from neurophysiological studies of the mustached bat (Pteronotus parnellii). This highly social and vocal species retrieves precise information about the velocity and range of its targets through echolocation. Such high acoustic processing demands were likely the evolutionary pressures driving the over-development at peripheral (cochlea), metencephalic (cochlear nucleus), mesencephalic (inferior colliculus), diencephalic (medial geniculate body of the thalamus), and telencephalic (auditory cortex) auditory processing levels in this species. Auditory researchers stand to benefit from a three dimensional brain atlas of this species, due to its considerable contribution to auditory neuroscience. Our MRI-based atlas was generated from 2 sets of image data of an ex-vivo male mustached bat’s brain: a detailed 3D-T2-weighted-RARE scan [(59 × 63 × 85) μm3] and track density images based on super resolution diffusion tensor images [(78) μm3] reconstructed from a set of low resolution diffusion weighted images using Super-Resolution-Reconstruction (SRR). By surface-rendering these delineations and extrapolating from cortical landmarks and data from previous studies, we generated overlays that estimate the locations of classic functional subregions within mustached bat auditory cortex. This atlas is freely available from our website and can simplify future electrophysiological, microinjection, and neuroimaging studies in this and related species.
Graphical Abstract
INTRODUCTION
The mustached bat (Pteronotus parnellii) is highly reliant on its auditory system for orientation and communication and has accordingly developed neural processing areas within its auditory system that are hypertrophic relative to its other brain structures (Baron, 1974). Studies of these hypertrophic auditory system nuclei provided substantial knowledge regarding the function of mammalian auditory systems at the peripheral (Xiao and Suga, 2002a), metencephalic (Kemmer and Vater, 1997), mesencephalic (Gordon and O’Neill, 2000; Portfors and Wenstrup, 1999; Wenstrup et al., 2012), diencephalic (Butman and Suga, 2016; Suga et al., 1997; Wenstrup, 1999), and telencephalic (O’Neill and Suga, 1979; Suga and Jen, 1976; Suga et al., 1979) levels.
The success of these studies was founded on the delimited behavioral context of echolocation, in which biologically relevant scientific questions about the auditory system could be formulated within a common theoretical framework (Ehret, 1997; Suga, 1985). During echolocation, these bats emit a sound consisting of a constant frequency (CF), a downward frequency modulation (FM), and three harmonics thereof. By emitting these four signals (fundamental + 3 harmonics), mustached bats are able to track both the relative velocities and ranges of targets via the returning echo CF and FM components, respectively. Simple and complex feature detectors at every level of the mustached bat auditory system are specialized to process such CF and FM components in parallel. Studies of such feature detectors led to the discovery of neuroacoustic phenomena in this species prior to their documentation in other mammals. At the cortical level, these findings include spectral and temporal combination-sensitivity (O’Neill and Suga, 1979; Suga et al., 1978, 1979), disproportionate tonotopic representation (Suga and Jen, 1976), and excitatory-excitatory/inhibitory-excitatory (EE/IE) binaural organization (Manabe et al., 1978). Similarly impactful findings at the subcortical level include systematic representation of interaural level differences in the ascending auditory system (Wenstrup et al., 1986), spectral and temporal integration in the inferior colliculus (Wenstrup et al., 2012), projections from the amygdala to the inferior colliculus (Marsh et al., 2002), and the nucleus of the central acoustic tract or NCAT (Casseday et al., 1989). Even studies of the neural substrates underlying social communication in the mustached bat auditory system were bolstered by knowledge gleaned from echolocation-based studies in the same species (Esser et al., 1997; Kanwal, 1999).
Neuroimaging techniques such as magnetic resonance imaging (MRI) have improved considerably in recent years with the availability of high field MRI scanners and thus would allow for detailed anatomical and functional studies spanning multiple nuclei and networks across the mustached bat central nervous system. Given the hypertrophy of auditory areas in the mustached bat, functional MRI studies could give insight into auditory processing between subcortical acoustic nuclei using non-invasive techniques that can be used repeatedly in an individual. The first in-vivo MRI study of the mustached bat brain confirmed that anatomical and auditory fMRI scans of this species can be achieved even in awake animals (Kamada et al., 1999). A number of MRI studies of cochlear and brain structure volumes in bats have recently emerged (Hsiao et al., 2016; Hsiao et al., 2015). Thus, any future functional neuroimaging studies in this species would benefit from a comprehensive reference such as a published digital mustached bat brain atlas. An MRI atlas of the mustached bat brain with labels for the gross structures of the auditory system as well as certain salient non-auditory brain structures and landmarks would represent a 3D-reference database for future neurophysiological, microinjection, and neuroimaging studies in this species. Detailed interpretations of data from such investigations on a neuroanatomical basis are possible with the aid of a cyto- and myeloarchitectonic mustached bat brain atlas currently under construction and congruent with MRI brain series of the mustached bat (Radtke-Schuller et al, unpublished data). Here, we present the freely downloadable MRI atlas of a male mustached bat brain, based on T2-weighted and track density images as well as published maps of the auditory cortex. The mustached bat brain atlas presented here can be easily adapted to match surgical and electrophysiological set ups as well as histological protocols, making it appealing for future bat brain studies.
METHODS
Specimen preparation
One adult male mustached bat (Pteronotus parnellii rubiginosus) was euthanized with an overdose of sodium pentobarbital (Fatal Plus, >100 mg/kg, i.p., Vortech, Dearborn, MI). Following loss of corneal and withdrawal reflexes, the animal was transcardially perfused through the left ventricle with phosphate buffered saline (PBS), followed by a 10% formalin mixture. After decapitation, the head was post-fixed in a mixture of 10% formalin for over 5 days at 20°C. The brain was never physically removed from the skull during MRI and CT data acquisition. Though significant brain shrinkage is generally expected due to fixation, successful registrations between anatomical MRIs of the brain and a CT image of the skull strongly suggest that brain shrinkage and/or malformation was minimal. A glass microelectrode was inserted while the bat was alive, targeted the medial geniculate body of the thalamus (MG), but caused no apparent damage to this particular structure. However, the electrode insertion caused some visible damage to other areas of the brain including the cortex, deep cerebral white matter, and hippocampus. As shown in the supplementary materials (Fig. SSF1), the neurological damage due to this electrode penetration was unilateral (right hemispheric), restricted to relatively few voxels contained within the aforementioned quasi-planar structures, could be compensated for during the delineation process via comparisons with the left hemisphere and histological sections (see below), and thus had negligible impact on the quality of our delineations. All specimen preparation procedures were approved by the Northeast Ohio Medical University’s Institutional Animal Care and Use Committee.
Data acquisition
To obtain a three-dimensional digital representation of the bat’s skull, CT scans of the entire head were acquired with a Siemens Inveon PET-CT (rotation steps = 360; degrees = 360; voltage = 80 keV; tube current = 500pA; exposure time = 300 ms; voxel size = [38μm]3; matrix = [512]3).
All MRI datasets of the mustached bat head were acquired with a 9.4 T Biospec horizontal bore MR scanner (Bruker BioSpin, Ettlingen, Germany), equipped with a 120 mm BGA12-S actively shielded gradient insert with a maximum gradient strength of 600 mT/m attached to an AVANCE-II Bruker console. Images were acquired with a Bruker cross-coil setup with a linear transmit volume coil and a four-channel receiver cryo-probe designed for imaging rat heads. Horizontal T2-weighted 3D images were acquired using a Rapid Acquisition with Relaxation Enhancement sequence (repetition time [TR] = 4500 ms; echo time [TE] = 33 ms; voxel size = (59 × 63 × 85) pm3; image matrix = [256 × 256 × 136]; RARE factor = 6; scan time = 14.3 h; number of averages = [2]), hereafter referred to as T2w 3D-RARE images.
Fifteen low resolution multi-slice 2D diffusion weighted (DW) SE data sets were acquired where each set was rotated around the phase-encoding direction (i.e., medio-lateral axis) at incremental steps of 12° for the purpose of super resolution reconstruction as described previously (Hamaide et al., 2016; Van Steenkiste et al., 2015). Each of these sets consisted of one non-diffusion weighted (b0) and 6 DW images (b = 2500 s/mm2) with following acquisition parameters: TR = 8000 ms; TE = 27 ms; FOV = (20×20) mm2, acquisition matrix = [256 × 183], image matrix = [256 × 256], in-plane resolution of (0.078 × 0.078) mm2, slice thickness = 0.38 mm, 38 slices, D = 14 ms; d = 6 ms, acquisition time = 2.84 h. In total, the 15 rotated DW datasets provided diffusion weighting along 90 unique diffusion gradient directions within a total scan-time of 42.7 h.
The dimensions of all of these images were ultimately cropped from 256 × 256 × 136 to 192 × 250 × 126 to remove excess space and improve visibility.
Data processing
Owing to the use of surface coils, bias field correction was applied to the T2w 3D-RARE. To perform super-resolution reconstruction diffusion tensor imaging (SRR-DTI), high resolution (78 μm)3 diffusion tensor parameter maps were estimated (Poot et al., 2010) from a set of low resolution diffusion weighted (DW) images using a weighted linear least squares estimator (Dyrby et al., 2014). From the high-resolution tensor parameters, high resolution fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) maps were obtained. High resolution fiber orientation distribution functions (fODFs) were also estimated in order to perform fiber tracking and subsequent track density imaging (TDI). First, a white matter fiber response function (spherical harmonic order 8) was extracted from the up-sampled high resolution DW data using the recursive calibration method (Tax et al., 2014). ODFs were obtained by performing constrained spherical deconvolution (Tournier et al., 2007), using the high resolution DW images and the single fiber response function created above, adopting the quadratic programming approach described previously (Jeurissen et al., 2014). The spherical harmonic series describing the fODFs was terminated at order eight. Next, probabilistic whole brain tractography was performed using second order integration over the fODFs (Tournier et al., 2010). Ten million streamlines were generated using the following parameters: fODF amplitude threshold of 0.1, step size of 40 μm, and a maximum angle between steps of 22°. Fidelity of the track densities to the raw diffusion weighted MRI data was ensured using SIFT2 (Smith et al., 2015). From the resulting tractogram, direction-encoded, color TDI maps were calculated at native resolution of (78 μm)3 (Calamante et al., 2010). All steps were performed using MRtrix3, which is freely downloadable at www.mrtrix.org (Tournier et al., 2012).
Delineation of Brain Structures
We choose to present all data in a 3D framework comparable to the orientation of Nissl and myelin stained histological slices of the histology atlas series provided by S.Radtke-Schuller. AMIRA 5.4.5 (Mercury Computer Systems, San Diego, CA, USA) was used to (1) rotate the T2w 3D-RARE scans to be consistent with the histological slices, (2) to perform the affine co-registration of TDI and CT images to the reoriented T2w 3D-RARE based on normalized mutual information, and (3) perform atlas delineations. The whole brain and brain regions of both hemispheres were delineated based on MRI contrasts between brain regions. TDIs were used in conjunction with the T2w 3D-RARE images to foster delineations by providing more accurate boundaries between gray and white matter. Delineations were made slice-by-slice in the three orthogonal dimensions: first anterior to posterior in the coronal dimension, then dorsal to ventral in the horizontal dimension, and then left to right in the sagittal dimension. Delineations made previously in one dimension (e.g., coronal) were systematically corrected as delineations progressed in a different dimension (e.g., horizontal).
The topology of forebrain (telencephalic and diencephalic) regions were identified on the basis of studies of the mustached bat brain (Fitzpatrick et al., 1998; Kobler et al., 1987; Pearson et al., 2007; Wenstrup et al., 1994; Winer and Wenstrup, 1994a, b; Winer et al., 1992) as well as a published atlas detailing structures in the forebrain of another bat species, Carollia perspicillata (Scalia et al., 2013). Identification of middle and hind brain (i.e., mesencephalic and metencephalic) structures were derived from studies in the mustached bat (Frisina et al., 1989; O’Neill et al., 1989; Ross and Pollak, 1989; Ross et al., 1988; Zook and Casseday, 1982a, b, 1985; Zook and Casseday, 1987; Zook et al., 1985). Further indications of boundaries were derived from histological brain atlases of rodents, including mice (Franklin and Paxinos, 2012), rats (Paxinos and Watson, 2014), and gerbils (Radtke-Schuller et al., 2016). The shapes of auditory cortical subregions were derived from maps reported in previously published histological and electrophysiological studies (Fitzpatrick et al., 1998; Suga, 1985). Such auditory cortex maps were superimposed onto a surface rendering of the mustached bat brain using the 3D rendering program Blender (https://www.blender.org). The resultant auditory cortical map can be viewed in MeshLab (http://www.meshlab.net/#download). Both Blender and MeshLab are a freely downloadable.
RESULTS
The MRI data set, structural delineations and CT images of the skull can be freely downloaded from our website (http://uahost.uantwerpen.be/bioimaginglab/Bat.zip). Data are provided in NIFTI (.nii) format. We also provide interactive templates (i.e., corresponding anatomical regions reported for each coordinate) viewable in two freely downloadable software packages, specifically MRIcro (http://people.cas.sc.edu/rorden/mricro/mricro.html#Installation) and MRIcron (http://people.cas.sc.edu/rorden/mricron/install.html). A third template is viewable in MRIcro-GL (https://www.nitrc.org/frs/?groupid=889), which measures inter-voxel distances at a sharper resolution of 0.1 mm and offers greater 3D rendering capabilities than either MRIcro or MRIcron. Each of these templates use color look-up-tables that match the color scheme used in the figures below. Viewing the delineations in other programs will yield different color schemes and will report only numerical values corresponding to intensities. Installers for Windows versions of each of these programs are included with our atlas. Mac OSX versions are available from the web addresses above.
We provide the data in a frame of reference consistent with mustached bat histological data (Radtke-Schuller et al, unpublished data). As opposed to rats and mice, the conventional brain axis tilt in mustached bats is 23°. The frontal atlas slicing plane was chosen to be perpendicular to the line that connects the most dorsal points of the forebrain (i.e., cortex) and hindbrain (i.e., cerebellum). This coordinate system is used for cutting and for the orientation of the bat’s skull in a stereotaxic device in neurophysiological experiments, as it allows for a more natural head-body orientation used in neurophysiological studies.
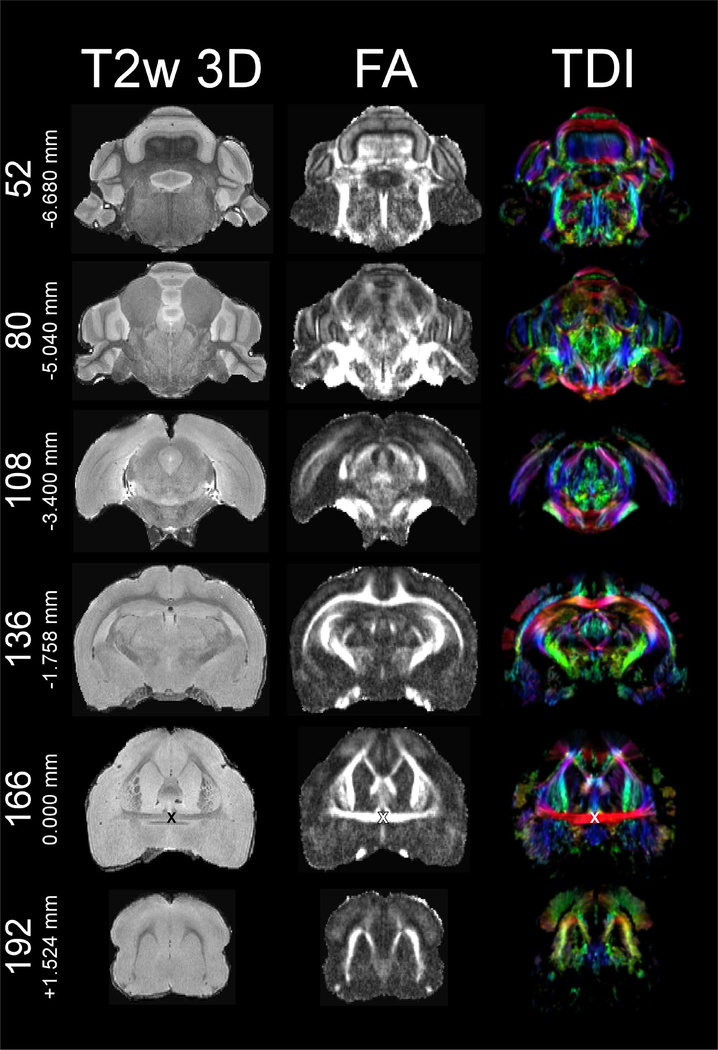
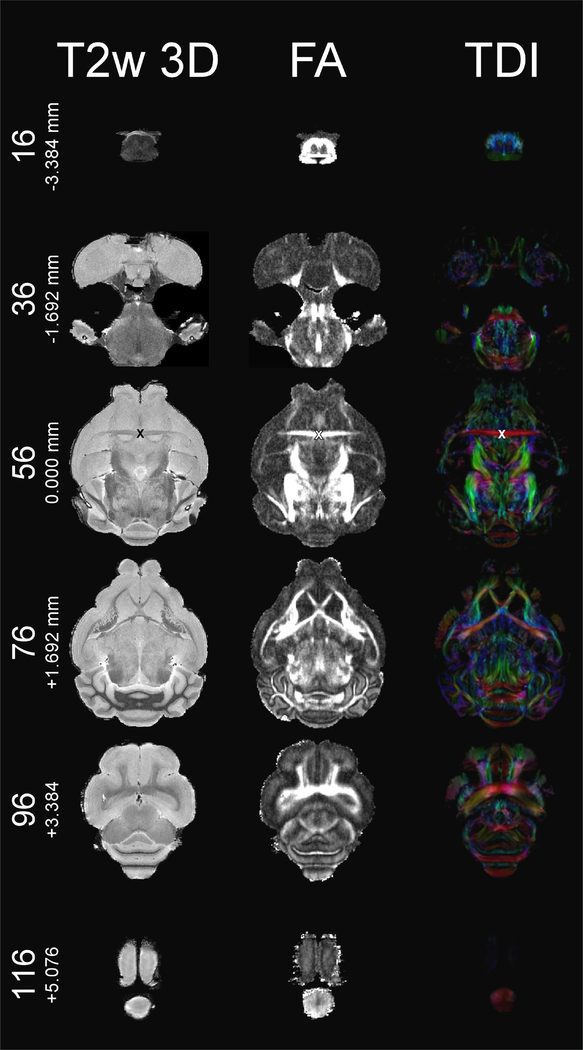
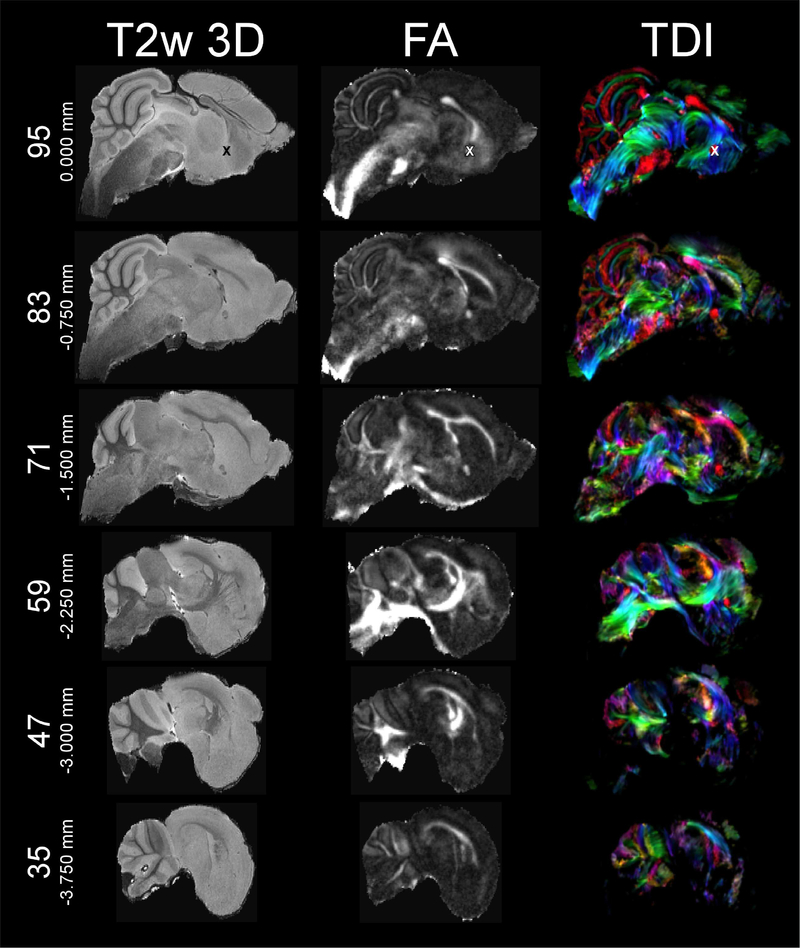
Various freely available imaging software packages enable users to reorient the frame of reference at will. Thus, users can visualize our data as either orthogonal or oblique slices. The anterior/posterior axis is the y-axis (positive values anterior), and the dorsal/ventral axis is the z-axis (positive values dorsal) in accordance with neurological convention (De Groof et al., 2016; Gunturkun et al., 2013; Poirier et al., 2008; Simoes et al., 2012), the left/right axis is the x-axis (positive values right). We propose the intersection between the mid-sagittal plane and the anterior commissure (ac) as the origin (i.e., zero-point) for the frame of reference due the ac’s similar role in the human-based Talairach coordinate system (Talairach and Tournoux, 1988) and the relative ease of locating ac in MRI images of the mustached bat brain. The origin of the dorsoventral plane is defined as the plane through the highest points of forebrain and cerebellum, which parallels the profile of the skull (see below). An “X” marks the origin in Figures 1, 2, and 3, each of which display six T2w 3D-RARE, FA, and TDI slices in the coronal, horizontal, and sagittal dimensions, respectively. Figures 1–3 are restricted to the T2w 3D-RARE, FA, and color-coded TDI maps due to space constraints. On the website, we additionally provide 3D maps of AD, MD, RD metrics, and b0 images in the same space.
Figure 1.
T2 weighted 3D-RARE (left), fractional anisotropy (middle), and color-coded tract density images (TDI) (right) at coronal planes 52/250 (−6.680 mm), 80/250 (−5.040 mm), 108/250 (−3.400 mm), 136/250 (−1.758 mm), 166/250 (0.000 mm), and 192/250 (+1.524 mm). “X” marks the origin (i.e., anterior-posterior zero point) at anterior commissure (plane 166/250) in all three modalities. Note that the apparent “shadow” in the left hemisphere of slice plane 108 in the T2w 3D-RARE map, which does not appear in the corresponding slice planes of the FA or color-coded TDI maps, is a signal void due to the relative local homogeneity of the magnetic field at the time of T2w 3D-RARE data acquisition. The diffusion-weighted scans (i.e., AD, b0, FA, MD, RD, and TDI) were acquired at a later time, had a better local shim, and thus did not present the same local signal void. Since the T2w 3D-RARE, the various diffusion-weighted scans, and histological sections were used as guides during delineation, our delineations of cortex were in no way complicated by this signal void. However, those using the T2w 3D-RARE map for their studies should be aware of this signal void.
Figure 2.
T2w 3D-RARE (left), FA (middle), and color-coded TDI (right) at horizontal planes 16/126 (−3.384 mm), 36/126 (−1.692 mm), 56/126 (0.000 mm), 76/126 (+1.692 mm), 96/126 (+3.384 mm), and 116/126 (+5.076 mm). “X” marks the origin at anterior commissure (plane 56/126) in all three modalities.
Figure 3.
T2w 3D-RARE (left), FA (middle), and color-coded TDI (right) at sagittal planes 95/192 (0.000 mm), 83/192 (−0.750 mm), 71/192 (−1.500 mm), 59/192 (−2.250 mm), 47/192 (−3.000 mm), and 35/192 (−3.750 mm). “X” marks the origin at anterior commissure (plane 95/192) in all three modalities. Since structures are largely similar between hemispheres, only left hemispheric slice planes are presented here.
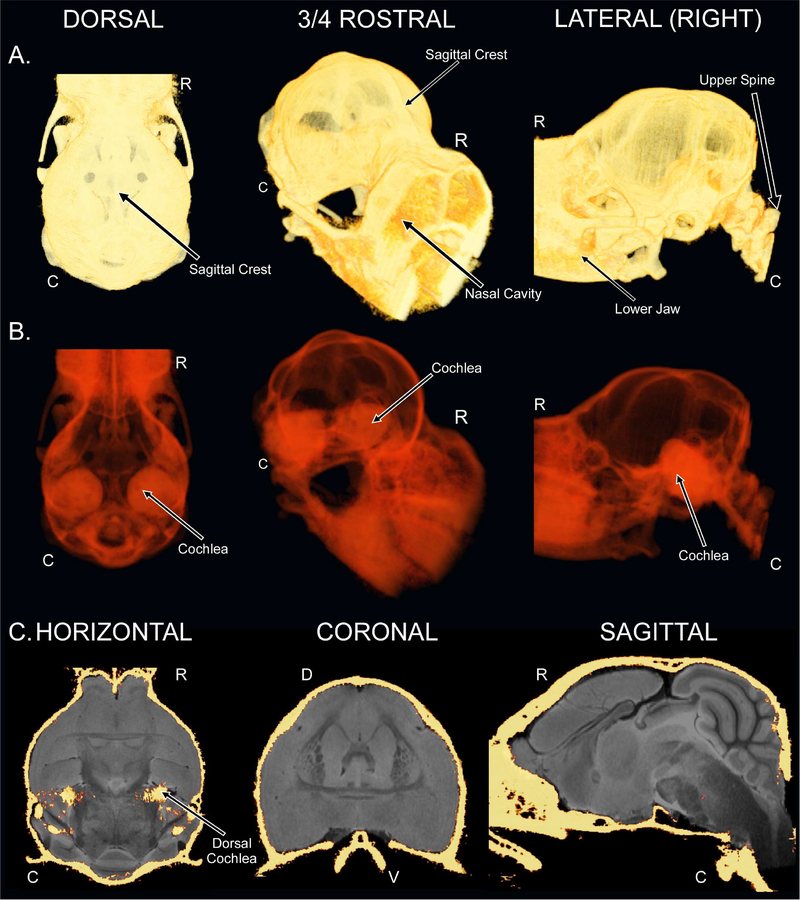
We also provide CT-based images of the bat skull co-registered to the MRI atlas. The CT images visualize the cochlea as more radiopaque than the neurocranium, demonstrating that the former structure has greater bone density than the latter in this species (Fig. 4A–B). Figure 4C displays the skull CT data set co-registered to the T2w 3D-RARE data set. Neurobiologists studying bats from a stereotaxic approach have previously established methodologies for employing landmarks on the skull as precise reference points for neural structures and functional areas (Schuller et al., 1991; Schuller et al., 1986). In brief, a coordinate system was previously developed for interfacing between stereotaxic procedures and histological processing of brains from another CF-FM bat species, the horseshoe bat, Rhinolophus rouxi (Schuller et al., 1986). As previously defined, these standard coordinate axes entailed orthogonal sagittal, coronal (frontal), and horizontal (parasagittal) skull planes (x = 0, y = 0, z = 0, respectively) in an electrode-carrier system. The zero-plane of the z-axis in this coordinate system coincides with the most dorsal points of the cortex and cerebellum. Researchers targeting structures in the mustached bat brain relative to the CT images provided are referred to the coordinate system detailed previously (Schuller et al., 1991). However, for those interested in comparing our skull CT to our MR-based images, the ac is also the zero-point of skull CT.
Figure 4.
CT-based images of the mustached bat skull and registration to the T2w 3D-RARE. (A) 3D renderings of the dorsal (left), three-quarter view rostral (middle), and right hemispheric lateral (right) views of the mustached bat skull. (B) 3D renderings of the mustached bat skull from the same perspectives as in “A” except that the radiopacity has been digitally reduced using the AlphaScale functionality of AMIRA 5.4.5. (C) Horizontal (left; slice 56/126, 0.000 mm), coronal (middle; slice 166/250, 0.000 mm), and sagittal (right; slice 95/192, 0.000 mm) co-registration of the CT images of the mustached bat skull and the brain-extracted T2w 3D-RARE images of the mustached bat brain. The bilateral bony structure located within the horizontal slice is the dorsal-most peak of the cochlea.
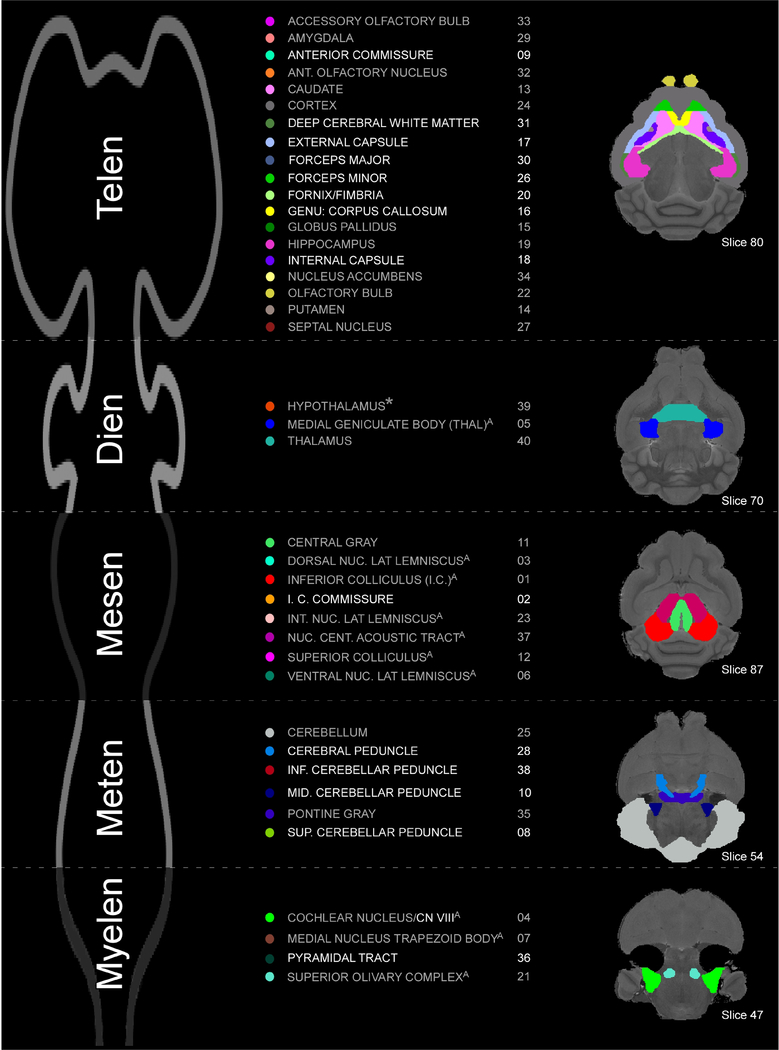
The T2w 3D-RARE enabled us to differentiate between different gray matter regions based on differences in T2-relaxation properties of distinct tissues. On the other hand, TDI and other diffusion images reveal axonal directions and other fine details of white matter (i.e., nerves and tracts) that are less apparent in the T2w 3D-RARE images. By using both types of images to better ascertain structural boundaries, we have delineated 40 different bilateral structures of interest in the mustached bat brain (Fig. 5). The column of numbers in Figure 5 correspond to intensity values of each delineation, and programs other than AMIRA 5.4.5, MRIcro, MRIcron, and MRIcro-GL will report these values as opposed to the corresponding brain region. Voxel counts, volumes, and centroids for all 40 delineated structures in the left and right hemispheres are summarized in Tables 1 and 2, respectively. Fourteen of these delineated structures were white matter. Of the remaining 26 structures, three were myelencephalic, two metencephalic, seven mesencephalic, three diencephalic, and 11 telencephalic. Ten of these 26 structures were part of the auditory system. We include the superior colliculus amongst these ten auditory structures. The superior colliculus is typically a multisensory area with a prevalent role in vision and oculomotor activity in highly visually oriented mammals. However, echolocating bats, including mustached bats, orient primarily using audition, not vision. Thus, neurons comprising significant portions of the superior colliculus in certain echolocating bat species, such as the horseshoe (Rhinolophus rouxi), big brown (Eptesicus fuscus), and pale spear-nosed (Phyllostomus discolor) bats, respond to auditory stimulation (Hoffmann et al., 2016; Reimer, 1991; Valentine and Moss, 1997; Wohlgemuth et al., 2018).
Figure 5.
Organization of brain delineations by embryonic subdivisions. (Left) An illustration of the basic embryonic organization of a mammalian brain separated into the telencephalic, diencephalic, mesencephalic, metencephalic, and myelencephalic subdivisions. (Middle) List of delineated regions per subdivision. Colored circles correspond to the colors of delineations at right and in subsequent figures but are specific to AMIRA 5.4.5. Numbers correspond to label color and are consistent between visualization programs (e.g., AMIRA, MRICron, AMIDE, etc.). Delineation text in gray represent gray matter structures and those in white represent white matter structures. Superscript “A” above a gray matter structure denotes that it is part of the auditory system. The asterisk (*) next to the hypothalamus indicates that, based on more recent developmental approaches, the hypothalamus is not included with other structures in the traditionally defined diencephalon (Puelles et al, 2013). (Right) Horizontal slices displaying delineations. Slices shown here were selected to display the greatest number of delineations per plane.
TABLE 1:
Left Hemispheric Measures
| Structure | Code | Count | Volume | X COM | Y COM | Z COM |
|---|---|---|---|---|---|---|
| Accessory Olfactory Bulb(AOB) | 33 | 303 | 0.094 | 9.014 | 13.057 | 6.328 |
| Amygdala(A) | 29 | 34350 | 10.637 | 11.023 | 9.258 | 4.005 |
| Anterior Commissure(ac) | 9 | 2932 | 0.908 | 9.310 | 10.270 | 5.468 |
| Anterior Olfactory Nucleus(AON) | 32 | 2390 | 0.740 | 9.121 | 12.580 | 5.435 |
| Caudate(C) | 13 | 27403 | 8.486 | 9.173 | 10.625 | 6.714 |
| Central Gray(CG) | 11 | 8807 | 2.727 | 8.284 | 5.920 | 7.701 |
| Cerebellum(Cb) | 25 | 224542 | 69.532 | 10.396 | 3.621 | 6.954 |
| Cerebral Peduncle(cp) | 28 | 3652 | 1.131 | 9.859 | 7.715 | 5.730 |
| Cochlear Nudeus/CNVMI(CN) | 4 | 10641 | 3.295 | 10.455 | 4.466 | 4.752 |
| Cortex(Neo) | 24 | 220732 | 68.352 | 10.684 | 9.243 | 7.613 |
| Deep Cereb White Matter(dcwm) | 31 | 8446 | 2.615 | 11.569 | 7.435 | 7.937 |
| Dorsal Nuc Lat Lemniscus(DNLL) | 3 | 745 | 0.231 | 9.654 | 5.199 | 6.838 |
| External Capsule(ec) | 17 | 22647 | 7.013 | 10.592 | 9.657 | 7.571 |
| Fimbria and Fornix Bundle(fi/f) | 20 | 5326 | 1.649 | 9.346 | 9.107 | 7.568 |
| Forceps Major (fmj) | 30 | 2956 | 0.915 | 9.237 | 7.633 | 9.472 |
| Forceps Minor(fmi) | 26 | 4435 | 1.373 | 9.354 | 11.720 | 7.079 |
| Genu: Corpus Callosum(gcc) | 16 | 11557 | 3.579 | 8.687 | 9.704 | 8.706 |
| Globus Pallidus(GP) | 15 | 2911 | 0.901 | 10.086 | 9.441 | 6.170 |
| Hippocampus(Hip) | 19 | 60488 | 18.731 | 10.871 | 7.504 | 6.530 |
| Hypothalamus(H) | 39 | 6930 | 2.146 | 8.552 | 9.121 | 4.248 |
| I.C. Commissure(cic) | 2 | 362 | 0.112 | 8.275 | 5.266 | 9.184 |
| Inferior Cerebellar Peduncle(icp) | 38 | 1251 | 0.387 | 9.658 | 4.040 | 6.677 |
| Inferior Colliculus(IC) | 1 | 26197 | 8.112 | 9.736 | 5.244 | 8.447 |
| Inter Nuc Lat Lemniscus(INLL) | 23 | 1961 | 0.607 | 10.033 | 5.455 | 6.462 |
| Internal Capsule(ic) | 18 | 13305 | 4.120 | 10.344 | 9.223 | 6.662 |
| Med Nuc Trapezoid Body(Tz) | 7 | 741 | 0.229 | 8.209 | 5.055 | 3.930 |
| Medial Cerebellar Peduncle(mcp) | 10 | 5031 | 1.558 | 10.490 | 5.116 | 5.831 |
| Medial Geniculate Body(MG) | 5 | 11215 | 3.473 | 10.394 | 7.621 | 7.000 |
| Nuc Cen Acoustic Tract(NCAT) | 37 | 201 | 0.062 | 8.937 | 5.759 | 4.886 |
| Nucleus Accumbens(Acb) | 34 | 3276 | 1.014 | 8.866 | 11.754 | 4.852 |
| Olfactory Bulb(OB) | 22 | 18178 | 5.629 | 8.978 | 13.575 | 6.350 |
| Pontine Gray(CGPn) | 35 | 6556 | 2.030 | 8.727 | 6.575 | 4.933 |
| Putamen(P) | 14 | 9746 | 3.018 | 10.466 | 9.306 | 5.542 |
| Pyramidal Tract(py) | 36 | 4008 | 1.241 | 8.322 | 4.196 | 3.029 |
| Septal Nucleus(S) | 27 | 3879 | 1.201 | 8.199 | 10.785 | 6.016 |
| Superior Cerebellar Peduncle(scp) | 8 | 364 | 0.113 | 9.187 | 4.678 | 6.920 |
| Superior Colliculus(SC) | 12 | 14571 | 4.512 | 8.913 | 6.864 | 8.296 |
| Superior Olivary Complex(SO) | 21 | 1699 | 0.526 | 8.966 | 5.061 | 4.662 |
| Thalamus(Thal) | 40 | 18200 | 5.636 | 8.841 | 8.831 | 6.772 |
| Ventral Nuc Lat Lemniscus(VNLL) | 6 | 2030 | 0.629 | 9.585 | 5.446 | 5.732 |
TABLE 2:
Right Hemispheric Measures
| Structure | Code | Count | Volume | X COM | Y COM | Z COM |
|---|---|---|---|---|---|---|
| Accessory Olfactory Bulb(AOB) | 33 | 244 | 0.08 | 6.681 | 12.987 | 6.379 |
| Amygdala(A) | 29 | 32281 | 10.00 | 4.747 | 9.253 | 4.072 |
| Anterior Commissure(ac) | 9 | 3010 | 0.93 | 6.564 | 10.234 | 5.479 |
| Anterior Olfactory Nucleus(AON) | 32 | 2634 | 0.82 | 6.683 | 12.588 | 5.529 |
| Caudate(C) | 13 | 25512 | 7.90 | 6.592 | 10.567 | 6.771 |
| Central Gray(CG) | 11 | 10687 | 3.31 | 7.604 | 6.009 | 7.643 |
| Cerebellum(Cb) | 25 | 224232 | 69.44 | 5.519 | 3.619 | 6.948 |
| Cerebral Peduncle(cp) | 28 | 3559 | 1.10 | 5.953 | 7.697 | 5.766 |
| Cochlear Nudeus/CNVMI(CN) | 4 | 10212 | 3.16 | 5.305 | 4.490 | 4.773 |
| Cortex(Neo) | 24 | 218009 | 67.51 | 5.218 | 9.261 | 7.682 |
| Deep Cereb White Matter(dcwm) | 31 | 9671 | 2.99 | 4.112 | 7.396 | 7.802 |
| Dorsal Nuc Lat Lemniscus(DNLL) | 3 | 709 | 0.22 | 6.160 | 5.190 | 6.814 |
| External Capsule(ec) | 17 | 23140 | 7.17 | 5.232 | 9.629 | 7.653 |
| Fimbria and Fornix Bundle(fi/f) | 20 | 5887 | 1.82 | 6.459 | 9.110 | 7.566 |
| Forceps Major (fmj) | 30 | 3079 | 0.95 | 6.572 | 7.620 | 9.505 |
| Forceps Minor(fmi) | 26 | 4573 | 1.42 | 6.447 | 11.676 | 7.027 |
| Genu: Corpus Callosum(gcc) | 16 | 12033 | 3.73 | 7.206 | 9.717 | 8.661 |
| Globus Pallidus(GP) | 15 | 2802 | 0.87 | 5.669 | 9.406 | 6.215 |
| Hippocampus(Hip) | 19 | 58969 | 18.26 | 4.920 | 7.475 | 6.596 |
| Hypothalamus(H) | 39 | 7288 | 2.26 | 7.271 | 9.156 | 4.281 |
| I.C. Commissure(cic) | 2 | 409 | 0.13 | 7.630 | 5.264 | 9.211 |
| Inferior Cerebellar Peduncle(icp) | 38 | 1161 | 0.36 | 6.085 | 4.026 | 6.662 |
| Inferior Colliculus(IC) | 1 | 27635 | 8.56 | 6.131 | 5.238 | 8.478 |
| Inter Nuc Lat Lemniscus(INLL) | 23 | 2090 | 0.65 | 5.729 | 5.410 | 6.477 |
| Internal Capsule(ic) | 18 | 14447 | 4.47 | 5.455 | 9.215 | 6.671 |
| Med Nuc Trapezoid Body(Tz) | 7 | 863 | 0.27 | 7.617 | 5.095 | 3.928 |
| Medial Cerebellar Peduncle(mcp) | 10 | 4204 | 1.30 | 5.433 | 5.215 | 5.857 |
| Medial Geniculate Body(MG) | 5 | 12288 | 3.81 | 5.305 | 7.593 | 7.058 |
| Nuc Cen Acoustic Tract(NCAT) | 37 | 185 | 0.06 | 7.007 | 5.768 | 4.822 |
| Nucleus Accumbens(Acb) | 34 | 3210 | 0.99 | 6.880 | 11.751 | 4.862 |
| Olfactory Bulb(OB) | 22 | 20924 | 6.48 | 6.916 | 13.563 | 6.324 |
| Pontine Gray(CGPn) | 35 | 6925 | 2.14 | 7.169 | 6.565 | 4.922 |
| Putamen(P) | 14 | 10197 | 3.16 | 5.307 | 9.268 | 5.491 |
| Pyramidal Tract(py) | 36 | 5095 | 1.58 | 7.545 | 4.147 | 3.000 |
| Septal Nucleus(S) | 27 | 5812 | 1.80 | 7.653 | 10.798 | 5.946 |
| Superior Cerebellar Peduncle(scp) | 8 | 357 | 0.11 | 6.618 | 4.635 | 6.898 |
| Superior Colliculus(SC) | 12 | 15218 | 4.71 | 6.922 | 6.871 | 8.320 |
| Superior Olivary Complex(SO) | 21 | 1714 | 0.53 | 6.801 | 5.030 | 4.693 |
| Thalamus(Thal) | 40 | 19887.00 | 6.16 | 7.033 | 8.843 | 6.792 |
| Ventral Nuc Lat Lemniscus(VNLL) | 6 | 2022.00 | 0.63 | 6.205 | 5.401 | 5.746 |
Maps derived from DW imaging were instrumental to delineating certain regions and boundaries that were simply not visible in the T2w 3D-RARE. More specifically, RD helped elucidate the medial boundaries of the amygdala, hippocampus, and medial geniculate body of the thalamus (MGB) as well as ventral extents of the external capsule. TDI enabled us to delineate the nucleus of the central acoustic tract (NCAT), the hypothalamus, and to more accurately delineate boundaries for white matter structures, including the ventral extents of the external capsule.
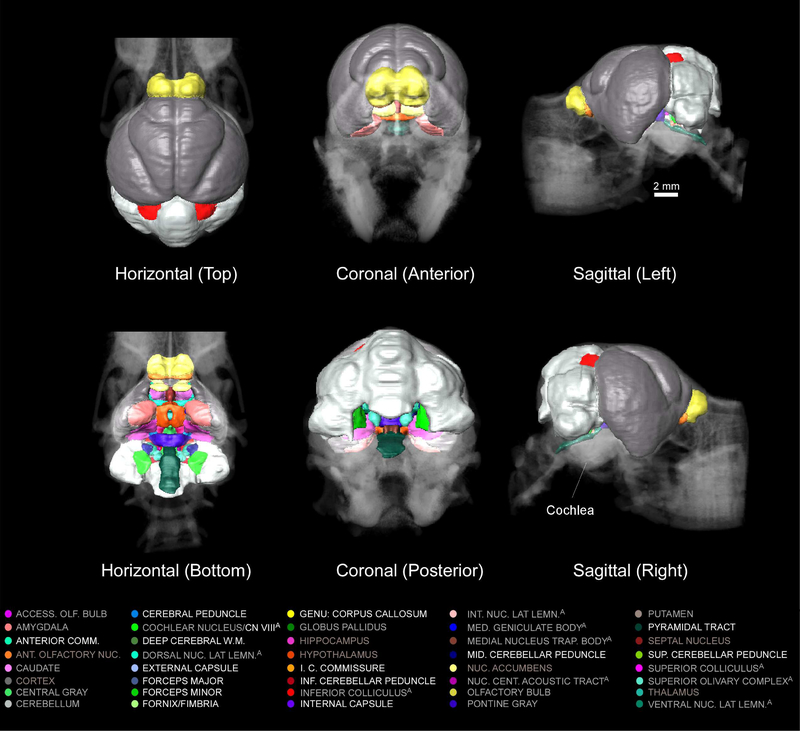
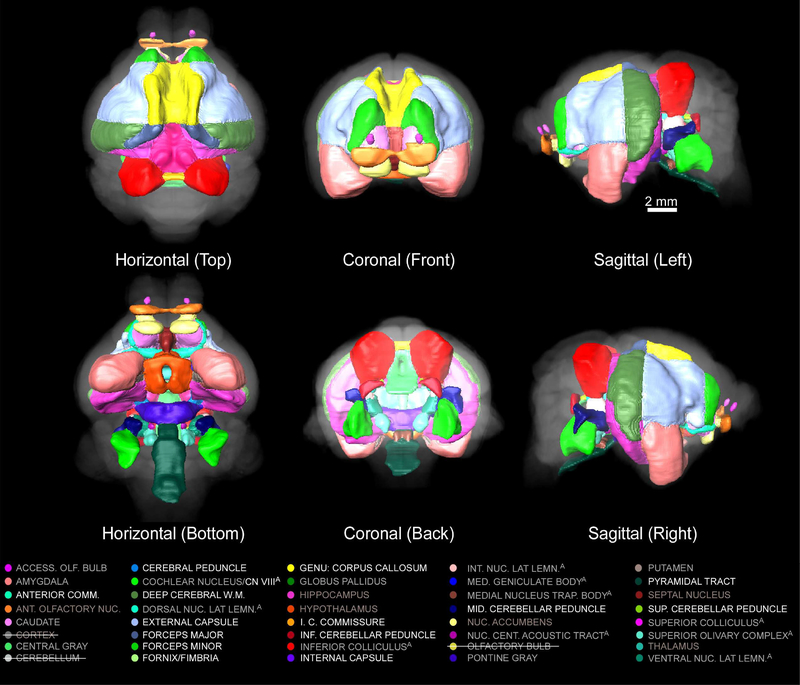
Three-dimensional renderings of delineated structures can be generated in AMIRA 5.4.5 or other software. Such three dimensional representations can provide a good approximation of both the shape and relative volume of each delineated structure. Three-dimensional renderings of these delineated structures are displayed within a transparent volume rendering of the skull (CT image) in the horizontal, coronal, and sagittal dimensions in Figure 6. Figure 7 displays similar 3D renderings of delineated structures but provides a clearer view of subcortical structures by excluding renderings of the cerebellum, cortex, and olfactory bulb.
Figure 6.
Three-dimensional rendering of brain delineations registered to the skull (CT) from the top-horizontal (top left), bottom-horizontal (bottom left), anterior-coronal (middle top), posterior-coronal (middle bottom), left-sagittal (top right), and right-sagittal (bottom right) perspectives. The cochlea is pointed out in the bottom-right panel. Color codes for each of the 40 delineated structures are provided below.
Figure 7.
Three-dimensional rendering of subcerebellar and subcortical delineations registered to the mustached bat brain (T2w 3D-RARE) from the top-horizontal (top left), bottom-horizontal (bottom left), anterior-coronal (middle top), posterior-coronal (middle bottom), left-sagittal (top right), and right-sagittal (bottom right) perspectives. The cerebellum, the cortex, and the olfactory bulb are excluded from the delineated structures shown here and in the color codes provided below to avoid obscuring subcerebellar and subcortical structures.
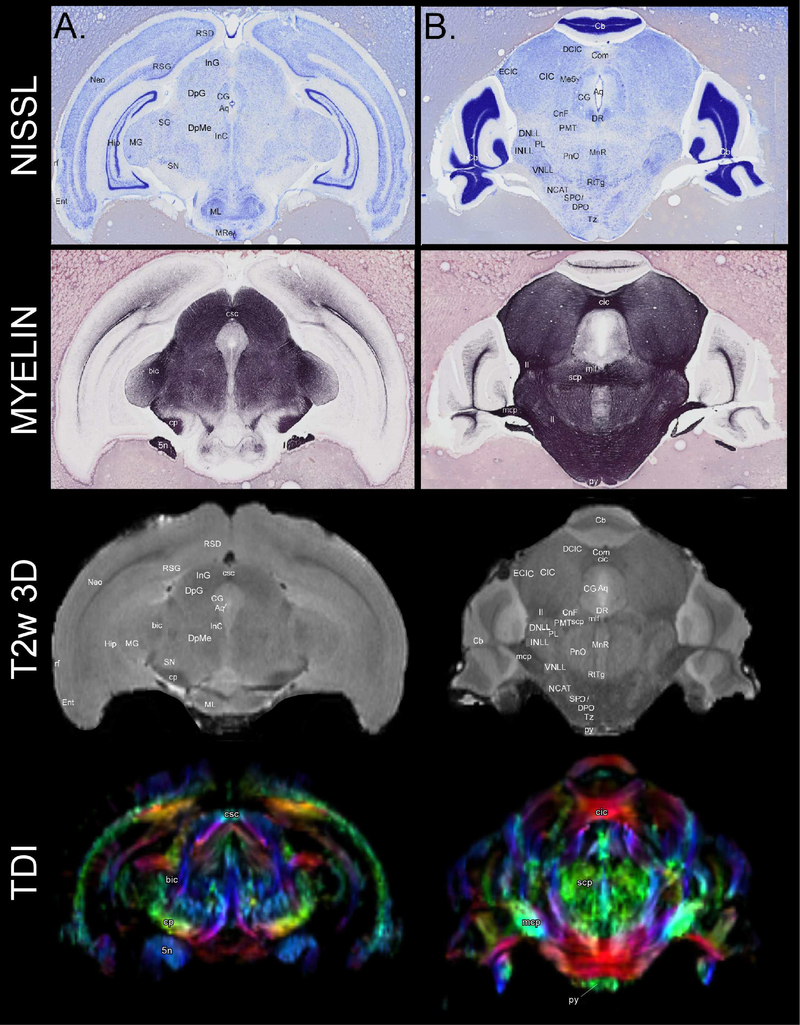
In Figure 8, Nissl and myelin stained sections of the histological atlas of the mustached bat are juxtaposed to our T2w 3D-RARE and TDI. Nissl and myelin stained histological slices have cellular level resolution, which surpasses the image resolution of the TDI and T2w 3D-RARE images. Thus, identification of structures in the Nissl and myelin stained slices can be used to label, and potentially digitally delineate, fine structures on MR-based images that would otherwise be difficult to discern. The labels on the T2w 3D-RARE in Figure 8 suggest the possibility of using histological reference slides to create even more precise labeling than those shown above.
Figure 8.
Comparisons between labeled Nissl and myelin stained coronal histological slices and coronal T2w 3D-RARE MR-based images. The Nissl and myelin stains derive from an unpublished neurological atlas of the mustached bat. (A) Nissl (top) and myelin (middle top) slices that correspond to the T2w 3D-RARE (middle bottom) and color-coded track density (bottom) images (120/250, −2.695 mm, coronal). Slice and image numbers are listed from anterior to posterior. (B) Nissl (top) and myelin (middle top) that correspond to the T2w 3D-RARE (middle bottom) and track density (bottom) images (88/250, −4.570 mm, coronal). Label abbreviations: 5n (trigeminal nerve), Aq (cerebral aqueduct), bic (brachium of the inferior colliculus), NCAT (nucleus of the central acoustic tract), Cb (cerebellum), CG (central gray), CIC (central nucleus of the inferior colliculus), cic (commissure of the inferior colliculus), CnF (cuneiform nucleus), Com (commissural nucleus of inferior colliculus), cp (cerebral peduncle), csc (commissure of the superior colliculus), DCIC (dorsal cortex of the inferior colliculus), DNLL (dorsal nucleus of lateral lemniscus), DpME (deep mesencephalic nucleus; also known as p1PAG), DpG (deep layers of superior colliculus), DPO (dorsomedial paraolivary nucleus), DR (dorsal raphe nucleus), ECIC (external cortex of the inferior colliculus), Ent (entorhinal area), Hip (hippocampus), INLL (intermediate nucleus of the lateral lemniscus), InC (interstitial nucleus of Cajal), InG (intermediate layers of superior colliculus), ll (lateral lemniscus), mcp (cerebellar peduncle), Me5 (mesencephalic trigeminal nucleus), MG (medial geniculate body), ML (lateral part of medial mammillary nucleus), mlf (medial longitudinal fasciculus), MnR (medial superior central raphe nucleus), MRe (mammillary recess of the 3rd ventricle), Neo (neocortex), PL (paralemniscal nucleus), PMT (pontomesencephalic tegmentum), PnO (pontine reticular nucleus, oral part), py (pyramidal tract), rf (rhinal fissure), RSD (dorsal retosplenial cortex), RSG (retrosplenial dysgranular cortex), RtTg (reticulotegmental nucleus of pons), scp (superior cerebellar peduncle), SG (suprageniculate thalamic body), SN (substantia nigra), SPO (superior paraolivary nucleus), Tz (medial nucleus of the trapezoid body), and VNLL (ventral nucleus of the lateral lemniscus). Each of the acronyms above are derived from Franklin and Paxinos (2012) except for certain large structures (i.e., Cb, Ent, Hip, Neo) that we chose not to delineate into smaller structures and the pontomesencephalic tegmentum, a structure that is still commonly referenced in literature (Schofield et al., 2011).
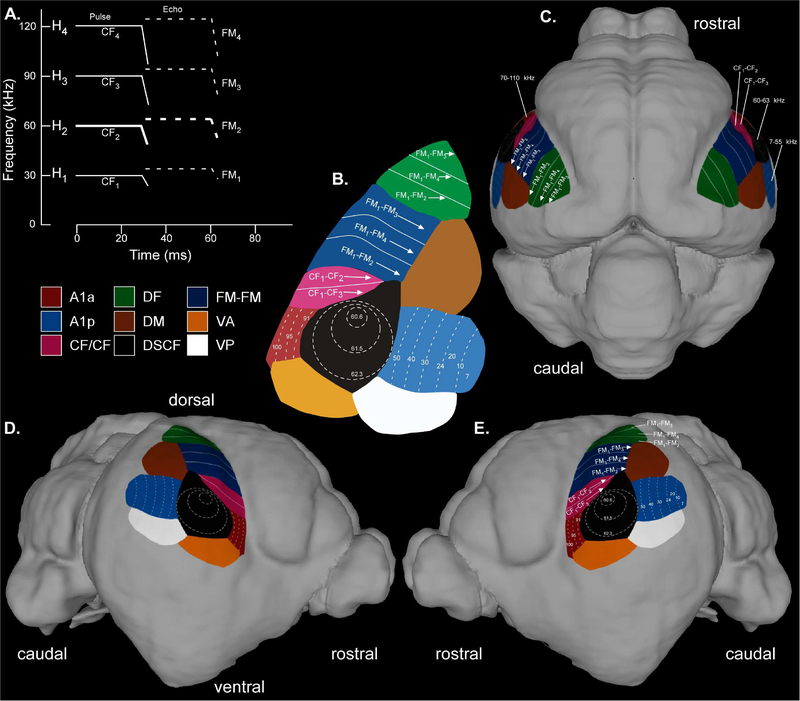
One of the most prominently studied portions of the mustached bat auditory system is the functional organization of its auditory cortex. Neurophysiological studies are impossible in exvivo brains. However, decades of neurophysiological and histological studies have linked the location of mustached bat auditory cortex and its subdivisions to key cortical features, which were revealed by surface renderings of our delineations. Specifically, cortical delineations revealed the lateral fissure plus a triangular bulge associated with the auditory cortex, and adjustments in alpha scale revealed the medial cerebral artery and its bifurcations, commonly used by physiologists to target the Doppler-shifted constant frequency (DSCF) area. By aligning (1) the dorsal-most edges of both the dorsal fringe functional area (or DF) with the lateral fissure, (2) the anterior edges of the A1 anterior, CF/CF, DF, FM-FM, and ventral anterior subdivisions with the lateral fissure, and (3) the middle-dorsal portion of the DSCF area with the first bifurcation of the medial cerebral artery, we generated the 3D auditory cortex overlays displayed in Figure 9. The landmarks used to create this visualization are detailed in the supplementary materials (Fig. SSF2). This representation is viewable using MeshLab but not AMIRA, MRIcro, MRIcron, or MRIcroGL as it is an overlay and not comparable to our other delineations. Given that the location of cortical functional areas has a high degree of inter-subject variability, the locations of these subdivisions must be regarded as reasonable approximations.
Figure 9.
3D rendering of the functional organization of the mustached bat auditory cortex. (A) Schematized spectrogram of the mustached bat’s echolocation signal. H1-H4 refer to harmonics 1–4 of the echolocation pulse or echo. Note the constant frequency (CF) and frequency-modulated (FM) components present in the pulse (solid line) and returning echo (dashed line). (B) Map of the mustached bat auditory cortex. The boundaries of auditory cortical subdivisions are derived from Fitzpatrick et al (1998), and white labels describing the function of auditory cortical subdivisions are derived from Suga (1985). (C) Dorsal view of the mustached bat brain with a superimposed auditory cortex map. The map includes anterior A1 (A1a, maroon), posterior A1 (A1p, light blue), CF/CF (pink), dorsal fringe (DF, green), dorsal medial (DM, brown), Doppler-shifted CF (DSCF, black), FM-FM (dark blue), ventral anterior (VA, orange), and ventral posterior (VP, white). (D) Right profile view of the mustached bat brain with a superimposed auditory cortex map. This depiction contains the same functional areas described above in “C.” (E) Left profile view of the mustached bat brain similar to the depiction in “D.” Note that the DSCF labels in this depiction (60–63 kHz) represent the more commonly reported echo-CF2 range of the P.p.parnellii subspecies (Suga and Jen, 1976), not the 57–60 kHz range of the Trinidadian P.p.rubiginosus subspecies to which this specimen belongs (Xiao and Suga, 2002b). The Meshlab file used to generate this image contains the color-coded boundaries and contours but none of the numerical values, arrows, or text shown here. A second Meshlab file is available without contours. Researchers can thus label these ranges to coincide with those of P.p.parnellii, P.p.rubiginosus, or their choice of mustached bat subspecies. Details regarding the landmarks used to overlay the auditory cortex map onto the surface rendering of the mustached bat brain are shown in Figure SSF2.
DISCUSSION
Brain atlases provide a reference framework for neuroscientists and have tremendous instructional value. Traditional brain atlases are based on histological sections with drawings of boundaries of structures and annotations (Franklin and Paxinos, 2012; Paxinos and Watson, 2014; Scalia et al., 2013). Currently, three-dimensional, digital MRI atlases are available for fish (Simoes et al., 2012; Ullmann et al., 2010), rodents (Bowden et al., 2011; Calabrese et al., 2013; Dorr et al., 2008; Johnson et al., 2012; Paxinos et al., 2015; Rumple et al., 2013; Valdes-Hernandez et al., 2011; Veraart et al., 2011), marsupials (Majka et al., 2013), songbirds and other aves (De Groof et al., 2016; Gunturkun et al., 2013; Poirier et al., 2008; Vellema et al., 2011), as well as humans and other primates (Bakker et al., 2015; Calabrese et al., 2015; Fan et al., 2016; Rohlfing et al., 2012). Detailed MRI-based atlases exist for regions of the mouse brain such as the basal ganglia (Ullmann et al., 2014) and diencephalon (Watson et al., 2017). Despite the remarkable contribution that studies of bats have made to our general understanding of auditory systems, the present atlas is the first MRI-based digital brain atlas of any echolocating bat species.
One clear limitation of this atlas is that it is based on an individual ex-vivo brain, similar to Talairach’s classic human brain atlas (Talairach and Tournoux, 1988). Thus, there is the possibility of inter-subject variability and researchers who require high spatial accuracy must consider this limitation when using this atlas. Similarly, shrinking effects due to fixation and potential neurological damage may increase inter-subject variability. However, as Figure 8 demonstrates, there is close correspondence between neuroanatomical structures seen in one bat’s MRI and another bat’s histological slices.
Although histological atlases generally have cellular-level resolution, MRI-based atlases have advantages for manipulating, analyzing, and normalizing neuroimaging data. Histological atlases are composed of frontal, horizontal, and sagittal sections from slicing three different brains, which implies that the three dimensional consistency cannot be totally asserted. Though inter-individual differences remain overall, the coronal, horizontal, and sagittal dimensions are consistent within individuals in an MRI atlas and permit acquisition of multiple MRI-based structural data types (e.g., T1, T2-weighted, diffusion tensor, etc.) from the same sample. Also, this 3D digital atlas allows users to present neuroanatomical data from the mustached bat from any oblique slice. The spatial relationship between gray and white matter is preserved in MRI-based atlases. Such three-dimensional, digital information will help researchers targeting deep brain structures (e.g., medial geniculate body of the thalamus, amygdala, cochlear nucleus, etc.) with electrodes, catheters, or injection micropipettes to perform experiments while minimizing damage to surrounding areas. If smaller, histologically distinguished sub-structures are targeted or if localized experiments should be functionally interpreted in detail, however, the additional use of a histological atlas will be essential. Yet, if it is possible to perform a 3D structural MRI scan on the animal prior to histological preparation, the MR images can be normalized to the present atlas in order to better specify the individual stereotaxic coordinates of a smaller ROI. In general, data from individual bats can be normalized to compensate for intersubject variability, which can allow for back-projection of ROIs into the native space of individual animals. Further, realistic adjustment of the sections to an MRI data set in the same cutting plane can substantially compensate for distortions, which can lead to a combined MRI/histology based atlas yielding high resolution at realistic coordinates, as demonstrated in the recently published cyto- and fiber architectonic atlas for the gerbil (Radtke-Schuller et al., 2016). Interestingly, though previous studies have detailed inter-species differences in bat cochlear size (Hsiao et al., 2015), the CT and MRI-based data obtained here offers cochlear dimensions for this particular, highly studied species in a format directly comparable to neurological structures such as the inferior colliculus. Also, the DWI-based data obtained here is the first to indicate the directionality of white matter tracts in any bat species. In general, MRI-based atlases make many different types of calculations upon brain structures possible, including group level measures of brain structure volume, connectivity strength, and stimulus/behavior driven brain activity, all of which can be easily visualized in three dimensions.
The present atlas compliments a commercially available histological atlas of a bat species, Carollia perspicillata or the short-tailed fruit bat (Scalia et al., 2013). This detailed atlas is limited only to forebrain structures and combined two histological staining techniques (i.e., Nissl and NeuN) that are ideal for labeling gray matter. Our atlas can aid those targeting mid-and hindbrain structures and used MRI techniques suited for both delineating gray and white matter structures. Further, though Carollia perspicillata and Pteronotus parnellii are both microchiroptera, or yangochiroptera (Hutcheon and Kirsch, 2006), their echolocation behavior differs dramatically. Specifically, the echolocation pulses of Carollia perspicillata consist only of FMs whereas those of Pteronotus parnellii consist of a tone followed by an FM (i.e., CF-FM). This vocal difference is already linked to differences in cochlear structure between FM-only and CF-FM bats (Hsiao et al., 2015). Considering the vast amount of neurophysiological, neuroanatomical, and behavioral studies in the mustached bat and the ongoing investigations in this bat species, an MRI-based atlas of the mustached bat brain is timely.
Over a decade of advances in neuroimaging has enabled researchers to assess auditory processing via functional magnetic resonance imaging (fMRI) in small animals, including mice (Yu et al., 2007; Yu et al., 2005), rats (Gao et al., 2015; Gao et al., 2014), and songbirds (Poirier et al., 2009; Van Ruijssevelt et al., 2013). This atlas could serve as the anatomical reference for manganese-enhanced and diffusion tensor imaging studies in the mustached bat as well. Indeed, atlases similar to this one were used in manganese-enhanced and diffusion tensor imaging to study cognition and neuroplasticity in starlings (De Groof et al., 2006; Van der Linden et al., 2004; Van Meir et al., 2006; Van Meir et al., 2004) and canaries (Tindemans et al., 2003). Manganese-enhanced imaging has even been successfully employed to study tonotopy in rodents (Yu et al., 2007). These and other voxel-based analyses in neuroimaging studies require precise spatial normalization to some anatomical frame of reference analogous to the Talairach or Montreal Neurological Institute (MNI) coordinate systems commonly used in human-based neuroimaging studies. Perhaps, the strongest contribution of this atlas may be the support it can offer future functional, structural, and physiological (e.g. perfusion) neuroimaging studies in this species. At the gross anatomical level, functional neuroimaging is poised to address long-standing auditory neuroscience controversies commonly explored by single unit recording studies in bats, including the corticofugal system (Yan and Suga, 1996; Zhang and Suga, 1997), echo-acoustic flow (Bartenstein et al., 2014; Greiter and Firzlaff, 2017), and left hemispheric specialization for processing social calls (Kanwal, 2012; Tallal, 2012; Washington and Kanwal, 2012; Washington and Tillinghast, 2015). This atlas is intended to serve as such an anatomical reference for future neuroimaging studies of the mustached bat brain, enabling researchers to accurately localize task, stimulus, or resting-state evoked blood oxygen level dependent (BOLD) signal changes related to neural activity in the forebrain, midbrain, and brainstem.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge Dr. Steven Staelens of the Molecular Imaging Center Antwerp for allowing us to use the Siemens Inveon PET-CT scanner to acquire the CT scans of the mustached bat brain. We also express our gratitude to Dr. Brent Harris of Georgetown University Medical Center, who helped cross-check and make suggestions regarding certain delineations, and Drs. Christophe Casteleyn and Marleen Cools of the University of Antwerp for their guidance with skull landmarks. We further express our gratitude to Drs. Chris Rorden and Kyle Shattuck of the University of South Carolina and Georgetown University Medical Center respectively for assisting us in formatting our images into interactive templates in the MRICro, MRICron, and MRICro-GL packages. We also thank Drs. Lawrence Kromer and Mark Burns of Georgetown University Medical Center for comments on this manuscript. The Ministry of Agriculture, Land and Marine Resources in Trinidad kindly permitted F. Muradali to collect and export mustached bats to Dr. Wenstrup’s laboratory in the US, from which the sample we ultimately used originated. This work was supported in part by an Erasmus Mundus Cognitive Auditory Neuroscience Exchange Program stipend awarded to S.D.W. J.J.W. was supported by NIH R01 DC00937 from the National Institute on Deafness and other Communications Disorders, of the United States Public Health Service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bakker R, Tiesinga P, Kotter R, 2015. The Scalable Brain Atlas: Instant Web-Based Access to Public Brain Atlases and Related Content. Neuroinformatics 13, 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron G, 1974. Differential phylogenetic development of the acoustic nuclei among chiroptera. Brain Behav Evol 9, 7–40. [DOI] [PubMed] [Google Scholar]
- Bartenstein SK, Gerstenberg N, Vanderelst D, Peremans H, Firzlaff U, 2014. Echo-acoustic flow dynamically modifies the cortical map of target range in bats. Nat Commun 5, 4668. [DOI] [PubMed] [Google Scholar]
- Bowden DM, Johnson GA, Zaborsky L, Green WD, Moore E, Badea A, Dubach MF, Bookstein FL, 2011. A symmetrical Waxholm canonical mouse brain for NeuroMaps. J Neurosci Methods 195, 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butman JA, Suga N, 2016. Synaptic mechanisms shaping delay-tuned combination-sensitivity in the auditory thalamus of mustached bats. Hear Res 331, 69–82. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Badea A, Coe CL, Lubach GR, Shi Y, Styner MA, Johnson GA, 2015. A diffusion tensor MRI atlas of the postmortem rhesus macaque brain. Neuroimage 117, 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese E, Badea A, Watson C, Johnson GA, 2013. A quantitative magnetic resonance histology atlas of postnatal rat brain development with regional estimates of growth and variability. Neuroimage 71, 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamante F, Tournier JD, Jackson GD, Connelly A, 2010. Track-density imaging (TDI): super-resolution white matter imaging using whole-brain track-density mapping. Neuroimage 53, 1233–1243. [DOI] [PubMed] [Google Scholar]
- Casseday JH, Kobler JB, Isbey SF, Covey E, 1989. Central acoustic tract in an echolocating bat: an extralemniscal auditory pathway to the thalamus. J Comp Neurol 287, 247–259. [DOI] [PubMed] [Google Scholar]
- De Groof G, George I, Touj S, Stacho M, Jonckers E, Cousillas H, Hausberger M, Gunturkun O, Van der Linden A, 2016. A three-dimensional digital atlas of the starling brain. Brain Struct Funct 221, 1899–1909. [DOI] [PubMed] [Google Scholar]
- De Groof G, Verhoye M, Van Meir V, Tindemans I, Leemans A, Van der Linden A, 2006. In vivo diffusion tensor imaging (DTI) of brain subdivisions and vocal pathways in songbirds. Neuroimage 29, 754–763. [DOI] [PubMed] [Google Scholar]
- Dorr AE, Lerch JP, Spring S, Kabani N, Henkelman RM, 2008. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage 42, 60–69. [DOI] [PubMed] [Google Scholar]
- Dyrby TB, Lundell H, Burke MW, Reislev NL, Paulson OB, Ptito M, Siebner HR, 2014. Interpolation of diffusion weighted imaging datasets. Neuroimage 103, 202–213. [DOI] [PubMed] [Google Scholar]
- Ehret G, 1997. The auditory cortex. J Comp Physiol A 181, 547–557. [DOI] [PubMed] [Google Scholar]
- Esser KH, Condon CJ, Suga N, Kanwal JS, 1997. Syntax processing by auditory cortical neurons in the FM-FM area of the mustached bat Pteronotus parnellii. Proceedings of the National Academy of Sciences of the USA 94, 14019–14024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Li H, zhuo J, Zhang Y, Wang J, Chen L, Yang Z, Chu C, Xie S, Laird AR, Fox PT, Eickhoff SB, Yu C, Jiang T, 2016. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb Cortex 26, 3508–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick DC, Olsen JF, Suga N, 1998. Connections among functional areas in the mustached bat auditory cortex. Journal of Comparative Neurology 391, 366–396. [PubMed] [Google Scholar]
- Franklin KJB, Paxinos G, 2012. The Mouse Brain in Stereotaxic Coordinates, 4th ed. Academic Press (Elsevier), New York, NY. [Google Scholar]
- Frisina RD, O’Neill WE, Zettel ML, 1989. Functional organization of mustached bat inferior colliculus: II. Connections of the FM2 region. J Comp Neurol 284, 85–107. [DOI] [PubMed] [Google Scholar]
- Gao PP, Zhang JW, Chan RW, Leong AT, Wu EX, 2015. BOLD fMRI study of ultrahigh frequency encoding in the inferior colliculus. Neuroimage 114, 427–437. [DOI] [PubMed] [Google Scholar]
- Gao PP, Zhang JW, Cheng JS, Zhou IY, Wu EX, 2014. The inferior colliculus is involved in deviant sound detection as revealed by BOLD fMRI. Neuroimage 91, 220–227. [DOI] [PubMed] [Google Scholar]
- Gordon M, O’Neill WE, 2000. An extralemniscal component of the mustached bat inferior colliculus selective for direction and rate of linear frequency modulations. J Comp Neurol 426, 165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiter W, Firzlaff U, 2017. Echo-acoustic flow shapes object representation in spatially complex acoustic scenes. J Neurophysiol 117, 2113–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunturkun O, Verhoye M, De Groof G, Van der Linden A, 2013. A 3-dimensional digital atlas of the ascending sensory and the descending motor systems in the pigeon brain. Brain Struct Funct 218, 269–281. [DOI] [PubMed] [Google Scholar]
- Hamaide J, De Groof G, Van Steenkiste G, Jeurissen B, Van Audekerke J, Naeyaert M, Van Ruijssevelt L, Cornil C, Sijbers J, Verhoye M, Van der Linden A, 2016. Exploring sex differences in the adult zebra finch brain: In vivo diffusion tensor imaging and ex vivo superresolution track density imaging. Neuroimage. [DOI] [PubMed] [Google Scholar]
- Hoffmann S, Vega-Zuniga T, Greiter W, Krabichler Q, Bley A, Matthes M, Zimmer C, Firzlaff U, Luksch H, 2016. Congruent representation of visual and acoustic space in the superior colliculus of the echolocating bat Phyllostomus discolor. Eur J Neurosci 44, 2685–2697. [DOI] [PubMed] [Google Scholar]
- Hsiao CJ, Hsu CH, Lin CL, Wu CH, Jen PH, 2016. Comparisons of MRI images, and auditory-related and vocal-related protein expressions in the brain of echolocation bats and rodents. Neuroreport. [DOI] [PubMed] [Google Scholar]
- Hsiao CJ, Jen PH, Wu CH, 2015. The cochlear size of bats and rodents derived from MRI images and histology. Neuroreport 26, 478–482. [DOI] [PubMed] [Google Scholar]
- Hutcheon JM, Kirsch JAW, 2006. A moveable face: deconstructing the Microchiroptera and a new classification of extant bats. Acta Chiropterologica 8, 1–10. [Google Scholar]
- Jeurissen B, Tournier JD, Dhollander T, Connelly A, Sijbers J, 2014. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage 103, 411–426. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Calabrese E, Badea A, Paxinos G, Watson C, 2012. A multidimensional magnetic resonance histology atlas of the Wistar rat brain. Neuroimage 62, 1848–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada K, Pekar JJ, Kanwal JS, 1999. Anatomical and functional imaging of the auditory cortex in awake mustached bats using magnetic resonance technology. Brain Res Brain Res Protoc 4, 351–359. [DOI] [PubMed] [Google Scholar]
- Kanwal JS, 1999. Processing species-specific calls by combination-sensitive neurons in an echolocating bat In: Hauser MD, Konishi M (Eds.), The Design of Animal Communication. The MIT Press, Cambridge, Ma, pp. 135–157. [Google Scholar]
- Kanwal JS, 2012. Right-left asymmetry in the cortical processing of sounds for social communication vs. navigation in mustached bats. Eur J Neurosci 35, 257–270. [DOI] [PubMed] [Google Scholar]
- Kemmer M, Vater M, 1997. The distribution of GABA and glycine immunostaining in the cochlear nucleus of the mustached bat (Pteronotus parnellii). Cell Tissue Res 287, 487–506. [DOI] [PubMed] [Google Scholar]
- Kobler JB, Isbey SF, Casseday JH, 1987. Auditory pathways to the frontal cortex of the mustache bat, pteronotus parnellii. Science 236, 824–826. [DOI] [PubMed] [Google Scholar]
- Majka P, Chlodzinska N, Banasik T, Djavadian RL, Węglarz WP, Turlejski K, Wojcik DK, 2013. Multimodal stereotactic template of the gray short-tailed opossum’s brain. Frontiers in Neuroinformatics, Stockholm, Sweden. [Google Scholar]
- Manabe T, Suga N, Ostwald J, 1978. Aural representation in the doppler-shifted-CF processing area of the auditory cortex of the mustache bat. Science 200, 339–342. [DOI] [PubMed] [Google Scholar]
- Marsh RA, Fuzessery ZM, Grose CD, Wenstrup JJ, 2002. Projection to the inferior colliculus from the basal nucleus of the amygdala. J Neurosci 22, 10449–10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill WE, Frisina RD, Gooler DM, 1989. Functional organization of mustached bat inferior colliculus: I. Representation of FM frequency bands important for target ranging revealed by 14C-2-deoxyglucose autoradiography and single unit mapping. Journal of Comparative Neurology 284, 60–84. [DOI] [PubMed] [Google Scholar]
- O’Neill WE, Suga N, 1979. Target range-sensitive neurons in the auditory cortex of the mustache bat. Science 203, 69–73. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2014. The Rat Brain in Stereotaxic Coordinates, 7th ed. Academic Press (Elsevier). [Google Scholar]
- Paxinos G, Watson C, Calabrese E, Badea A, Johnson G, 2015. MRI/DTI Atlas of the Rat Brain. Elsevier. [Google Scholar]
- Pearson JM, Crocker WD, Fitzpatrick DC, 2007. Connections of functional areas in the mustached bat’s auditory cortex with the auditory thalamus. J Comp Neurol 500, 401–418. [DOI] [PubMed] [Google Scholar]
- Poirier C, Boumans T, Verhoye M, Balthazart J, Van der Linden A, 2009. Own-song recognition in the songbird auditory pathway: selectivity and lateralization. J Neurosci 29, 2252–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier C, Vellema M, Verhoye M, Van Meir V, Wild JM, Balthazart J, Van Der Linden A, 2008. A three-dimensional MRI atlas of the zebra finch brain in stereotaxic coordinates. Neuroimage 41, 1–6. [DOI] [PubMed] [Google Scholar]
- Poot DH, Van Meir V, Sijbers J, 2010. General and efficient super-resolution method for multi-slice MRI. Med Image Comput Comput Assist Interv 13, 615–622. [DOI] [PubMed] [Google Scholar]
- Portfors CV, Wenstrup JJ, 1999. Delay-tuned neurons in the inferior colliculus of the mustached bat: implications for analyses of target distance. Journal of Neurophysiology 82, 1326–1338. [DOI] [PubMed] [Google Scholar]
- Radtke-Schuller S, Schuller G, Angenstein F, Grosser OS, Goldschmidt J, Budinger E, 2016. Brain atlas of the Mongolian gerbil (Meriones unguiculatus) in CT/MRI-aided stereotaxic coordinates. Brain Struct Funct 221 Suppl 1, 1–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer K, 1991. Auditory properties of the superior colliculus in the horseshoe bat, Rhinolophus rouxi. J Comp Physiol A 169, 719–728. [DOI] [PubMed] [Google Scholar]
- Rohlfing T, Kroenke CD, Sullivan EV, Dubach MF, Bowden DM, Grant KA, Pfefferbaum A, 2012. The INIA19 Template and NeuroMaps Atlas for Primate Brain Image Parcellation and Spatial Normalization. Front Neuroinform 6, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LS, Pollak GD, 1989. Differential ascending projections to aural regions in the 60 kHz contour of the mustache bat’s inferior colliculus. J Neurosci 9, 2819–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LS, Pollak GD, Zook JM, 1988. Origin of ascending projections to an isofrequency region of the mustache bat’s inferior colliculus. J Comp Neurol 270, 488–505. [DOI] [PubMed] [Google Scholar]
- Rumple A, McMurray M, Johns J, Lauder J, Makam P, Radcliffe M, Oguz I, 2013. 3-dimensional diffusion tensor imaging (DTI) atlas of the rat brain. PLoS One 8, e67334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalia F, Rasweiler JJ IV, Scalia J, Orman R, Stewart MG, 2013. Forebrain Atlas of the Short-tailed Fruit Bat, Carollia perspicillata: Prepared by the Methods of Nissl and NeuN Immunohistochemistry. Springer-Verlag, New York, NY [Google Scholar]
- Schofield BR, Motts SD, Mellott JG, 2011. Cholinergic cells of the pontomesencephalic tegmentum: connections with auditory structures from cochlear nucleus to cortex. Hear Res 279, 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller G, O’Neill WE, Radtke-Schuller S, 1991. Facilitation and Delay Sensitivity of Auditory Cortex Neurons in CF -FM Bats, Rhinolophus rouxi and Pteronotus p.parnellii. Eur J Neurosci 3, 1165–1181. [DOI] [PubMed] [Google Scholar]
- Schuller G, Radtke-Schuller S, Betz M, 1986. A stereotaxic method for small animals using experimentally determined reference profiles. J Neurosci Methods 18, 339–350. [DOI] [PubMed] [Google Scholar]
- Simoes JM, Teles MC, Oliveira RF, Van der Linden A, Verhoye M, 2012. A three-dimensional stereotaxic MRI brain atlas of the cichlid fish Oreochromis mossambicus. PLoS One 7, e44086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, Tournier JD, Calamante F, Connelly A, 2015. SIFT2: Enabling dense quantitative assessment of brain white matter connectivity using streamlines tractography. Neuroimage 119, 338–351. [DOI] [PubMed] [Google Scholar]
- Suga N, 1985. The extent to which biosonar information is represented in the bat auditory cortex In: Anderson JA, Pellionisz A, Rosenfeld E (Eds.), Neurocomputing 2: Directions for Research. MIT Press, Cambridge, MA, pp. 259–294. [Google Scholar]
- Suga N, Jen PH, 1976. Disproportionate tonotopic representation for processing CF-FM sonar signals in the mustache bat auditory cortex. Science 194, 542–544. [DOI] [PubMed] [Google Scholar]
- Suga N, O’Neill WE, Manabe T, 1978. Cortical neurons sensitive to combinations of information-bearing elements of biosonar signals in the mustache bat. Science 200, 778–781. [DOI] [PubMed] [Google Scholar]
- Suga N, O’Neill WE, Manabe T, 1979. Harmonic-sensitive neurons in the auditory cortex of the mustache bat. Science 203, 270–274. [DOI] [PubMed] [Google Scholar]
- Suga N, Zhang Y, Yan J, 1997. Sharpening of frequency tuning by inhibition in the thalamic auditory nucleus of the mustached bat. Journal of Neurophysiology 77, 2098–2114. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P, 1988. Co-Planar Stereotaxic Atlas of the Human Brain: 3-D Proportional System: An Approach to Cerebral Imaging. Thieme. [Google Scholar]
- Tallal P, 2012. Of bats and men. J Neurophysiol 108, 1545–1547. [DOI] [PubMed] [Google Scholar]
- Tax CM, Jeurissen B, Vos SB, Viergever MA, Leemans A, 2014. Recursive calibration of the fiber response function for spherical deconvolution of diffusion MRI data. Neuroimage 86, 67–80. [DOI] [PubMed] [Google Scholar]
- Tindemans I, Verhoye M, Balthazart J, Van Der Linden A, 2003. In vivo dynamic ME-MRI reveals differential functional responses of RA-and area X-projecting neurons in the HVC of canaries exposed to conspecific song. Eur J Neurosci 18, 3352–3360. [DOI] [PubMed] [Google Scholar]
- Tournier JD, Calamante F, Connelly A, 2007. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage 35, 1459–1472. [DOI] [PubMed] [Google Scholar]
- Tournier JD, Calamante F, Connelly A, 2010. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions Proceedings of the International Society Magnetic Resonance in Medicine
- Tournier JD, Calamante F, Connelly A, 2012. MRtrix: Diffusion tractography in crossing fiber regions. International Journal of Imaging Systems and Technology 22, 53–66. [Google Scholar]
- Ullmann JF, Cowin G, Kurniawan ND, Collin SP, 2010. A three-dimensional digital atlas of the zebrafish brain. Neuroimage 51, 76–82. [DOI] [PubMed] [Google Scholar]
- Ullmann JF, Watson C, Janke AL, Kurniawan ND, Paxinos G, Reutens DC, 2014. An MRI atlas of the mouse basal ganglia. Brain Struct Funct 219, 1343–1353. [DOI] [PubMed] [Google Scholar]
- Valdes-Hernandez PA, Sumiyoshi A, Nonaka H, Haga R, Aubert-Vasquez E, Ogawa T, Iturria-Medina Y, Riera JJ, Kawashima R, 2011. An in vivo MRI Template Set for Morphometry, Tissue Segmentation, and fMRI Localization in Rats. Front Neuroinform 5, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine DE, Moss CF, 1997. Spatially selective auditory responses in the superior colliculus of the echolocating bat. J Neurosci 17, 1720–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Linden A, Van Meir V, Tindemans I, Verhoye M, Balthazart J, 2004. Applications of manganese-enhanced magnetic resonance imaging (MEMRI) to image brain plasticity in song birds. NMR Biomed 17, 602–612. [DOI] [PubMed] [Google Scholar]
- Van Meir V, Pavlova D, Verhoye M, Pinxten R, Balthazart J, Eens M, Van der Linden A, 2006. In vivo MR imaging of the seasonal volumetric and functional plasticity of song control nuclei in relation to song output in a female songbird. Neuroimage 31, 981–992. [DOI] [PubMed] [Google Scholar]
- Van Meir V, Verhoye M, Absil P, Eens M, Balthazart J, Van der Linden A, 2004. Differential effects of testosterone on neuronal populations and their connections in a sensorimotor brain nucleus controlling song production in songbirds: a manganese enhanced-magnetic resonance imaging study. Neuroimage 21, 914–923. [DOI] [PubMed] [Google Scholar]
- Van Ruijssevelt L, Van der Kant A, De Groof G, Van der Linden A, 2013. Current state-of-the-art of auditory functional MRI (fMRI) on zebra finches: technique and scientific achievements. J Physiol Paris 107, 156–169. [DOI] [PubMed] [Google Scholar]
- Van Steenkiste G, Jeurissen B, Veraart J, den Dekker AJ, Parizel PM, Poot DH, Sijbers J, 2015. Super-resolution reconstruction of diffusion parameters from diffusion-weighted images with different slice orientations. Magn Reson Med. [DOI] [PubMed] [Google Scholar]
- Vellema M, Verschueren J, Van Meir V, Van der Linden A, 2011. A customizable 3-dimensional digital atlas of the canary brain in multiple modalities. Neuroimage 57, 352–361. [DOI] [PubMed] [Google Scholar]
- Veraart J, Leergaard TB, Antonsen BT, Van Hecke W, Blockx I, Jeurissen B, Jiang Y, Van der Linden A, Johnson GA, Verhoye M, Sijbers J, 2011. Population-averaged diffusion tensor imaging atlas of the Sprague Dawley rat brain. Neuroimage 58, 975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington SD, Kanwal JS, 2012. Sex-dependent hemispheric asymmetries for processing frequency-modulated sounds in the primary auditory cortex of the mustached bat. J Neurophysiol 108, 1548–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington SD, Tillinghast JS, 2015. Conjugating time and frequency: hemispheric specialization, acoustic uncertainty, and the mustached bat. Front Neurosci 9, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C, Janke AL, Hamalainen C, Bagheri SM, Paxinos G, Reutens DC, Ullmann JFP, 2017. An ontologically consistent MRI-based atlas of the mouse diencephalon. Neuroimage 157, 275–287. [DOI] [PubMed] [Google Scholar]
- Wenstrup JJ, 1999. Frequency organization and responses to complex sounds in the medial geniculate body of the mustached bat. Journal of Neurophysiology 82, 2528–2544. [DOI] [PubMed] [Google Scholar]
- Wenstrup JJ, Larue DT, Winer JA, 1994. Projections of physiologically defined subdivisions of the inferior colliculus in the mustached bat: targets in the medial geniculate body and extrathalamic nuclei. J Comp Neurol 346, 207–236. [DOI] [PubMed] [Google Scholar]
- Wenstrup JJ, Nataraj K, Sanchez JT, 2012. Mechanisms of spectral and temporal integration in the mustached bat inferior colliculus. Front Neural Circuits 6, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup JJ, Ross LS, Pollak GD, 1986. Binaural response organization within a frequency-band representation of the inferior colliculus: implications for sound localization. J Neurosci 6, 962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Wenstrup JJ, 1994a. Cytoarchitecture of the medial geniculate body in the mustached bat (Pteronotus parnellii). J Comp Neurol 346, 161–182. [DOI] [PubMed] [Google Scholar]
- Winer JA, Wenstrup JJ, 1994b. The neurons of the medial geniculate body in the mustached bat (Pteronotus parnellii). Journal of Comparative Neurology 346, 183–206. [DOI] [PubMed] [Google Scholar]
- Winer JA, Wenstrup JJ, Larue DT, 1992. Patterns of GABAergic immunoreactivity define subdivisions of the mustached bat’s medial geniculate body. J Comp Neurol 319, 172–190. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth MJ, Kothari NB, Moss CF, 2018. Functional Organization and Dynamic Activity in the Superior Colliculus of the Echolocating Bat, Eptesicus fuscus. J Neurosci 38, 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Suga N, 2002a. Modulation of cochlear hair cells by the auditory cortex in the mustached bat. Nature Neuroscience 5, 57–63. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Suga N, 2002b. Reorganization of the cochleotopic map in the bat’s auditory system by inhibition. Proc Natl Acad Sci U S A 99, 15743–15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Suga N, 1996. Corticofugal modulation of time-domain processing of bisonar information in bats. Science 273, 1100–1103. [DOI] [PubMed] [Google Scholar]
- Yu X, Sanes DH, Aristizabal O, Wadghiri YZ, Turnbull DH, 2007. Large-scale reorganization of the tonotopic map in mouse auditory midbrain revealed by MRI. Proc Natl Acad Sci U S A 104, 12193–12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Wadghiri YZ, Sanes DH, Turnbull DH, 2005. In vivo auditory brain mapping in mice with Mn-enhanced MRI. Nat Neurosci 8, 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Suga N, 1997. Corticofugal amplification of subcortical responses to single tone stimuli in the mustached bat. Journal of Neurophysiology 78, 3489–3492. [DOI] [PubMed] [Google Scholar]
- Zook JM, Casseday JH, 1982a. Cytoarchitecture of auditory system in lower brainstem of the mustache bat, Pteronotus parnellii. J Comp Neurol 207, 1–13. [DOI] [PubMed] [Google Scholar]
- Zook JM, Casseday JH, 1982b. Origin of ascending projections to inferior colliculus in the mustache bat, Pteronotus parnellii. J Comp Neurol 207, 14–28. [DOI] [PubMed] [Google Scholar]
- Zook JM, Casseday JH, 1985. Projections from the cochlear nuclei in the mustache bat, Pteronotus parnellii. J Comp Neurol 237, 307–324. [DOI] [PubMed] [Google Scholar]
- Zook JM, Casseday JH, 1987. Convergence of ascending pathways at the inferior colliculus of the mustache bat, pteronotus parnellii. Journal of Comparative Neurology 261, 347–361. [DOI] [PubMed] [Google Scholar]
- Zook JM, Winer JA, Pollak GD, Bodenhamer RD, 1985. Topology of the central nucleus of the mustache bat’s inferior colliculus: correlation of single unit properties and neuronal architecture. J Comp Neurol 231, 530–546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.