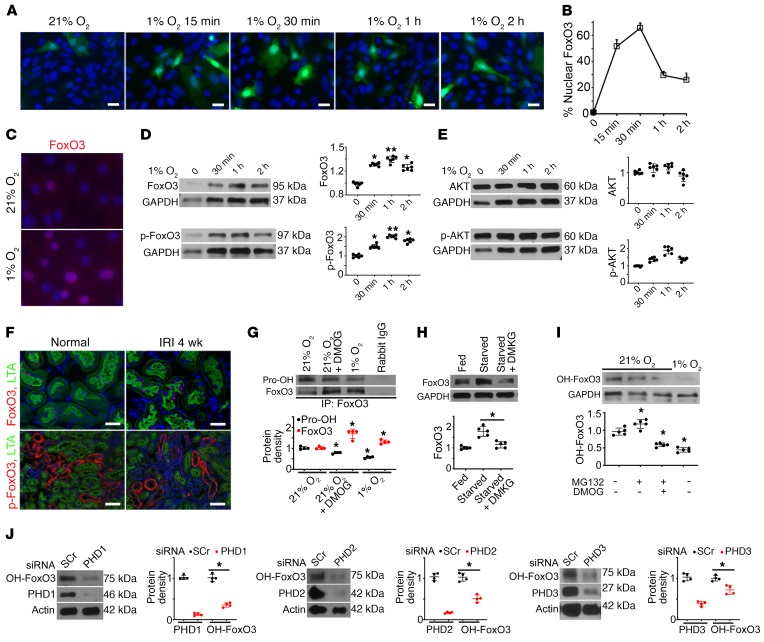
Figure 2. Hypoxia inhibits FoxO3 prolyl hydroxylation and its degradation.
(A–B) Exposure of primary cultures of renal epithelial cells infected with adeno-FoxO3-GFP to 1% O2 induced nuclear accumulation of FoxO3. (C) Immunostaining of endogenous FoxO3 (without overexpression) showed abundant nuclear FoxO3 (red) in cells exposed to 1% O2 for 30 minutes. (D and E) Endogenous FoxO3 and p-FoxO3 (Ser253) protein levels increased in primary cultures exposure to 1% O2, whereas no significant differences in AKT or p-AKT were detected. n = 6 with duplicates. *P ˂ 0.05 and **P ˂ 0.01 compared with 21% O2. (F) Proximal tubules of the kidney (labeled by LTA in green at the brush border) had increased FoxO3 expression in the nucleus (red), but undetectable p-FoxO3 in the cytoplasm (red) 4 weeks after IRI. n = 4 for normal controls; n = 5 for 4 weeks after IRI (IRI 4 wk). (G) IP of proteins isolated from primary cultures with an antibody against FoxO3 followed by IB analysis with a pan-hydroxyl proline antibody showed reduced prolyl hydroxylated proteins (Pro-OH) but increased FoxO3 protein expression in cells exposed to 1% O2 or cells grown in 21% O2 and treated with the hypoxia mimetic DMOG, which inhibited PHD enzymes. Rabbit IgG served as a control. n = 4. *P ˂ 0.05 compared with cells exposed to 21% O2 alone. (H) Supplementation of starved cells with DMKG, the cell-permeable analog of α-ketoglutarate, resulted in decreased FoxO3 protein abundance. n = 5. *P ˂ 0.05 comparing starved cells with starved cells plus DMKG. (I) An antibody raised against prolyl hydroxylated FoxO3 at Pro437 was used to detect OH-FoxO3 in primary cultures. OH-FoxO3 was present under 21% O2, increased when MG132 was used to block its degradation via ubiquitin degradation pathway, and decreased when treated with the combination of MG132 and the PHD enzyme inhibitor DMOG or exposure to 1% O2. n = 3 for rabbit IgG controls; n = 5 with duplicates for experimental conditions. *P ˂ 0.05 compared with 21% O2. (J) Knockdown of PHD1 (>80%), PHD2 (>80%), or PHD3 (>60%) with siRNA resulted in a significant reduction of OH-FoxO3 proteins in primary renal epithelial cells cultured under 21% O2 conditions. n = 4 with duplicates. *P ˂ 0.05 compared with cells transfected with scrambled siRNA (SCr). GAPDH served as a loading control in D, E, and H, and actin served as a loading control in J. Nuclei were counterstained with DAPI (blue) in A, C, and F. Scale bars: 20 μm (A and C) and 50 μm (F). A 2-tailed Student’s t test was performed for E, G, and J. A 1-way ANOVA followed by Dunnett’s post hoc test for multiple comparisons test was performed for D, H, and I.