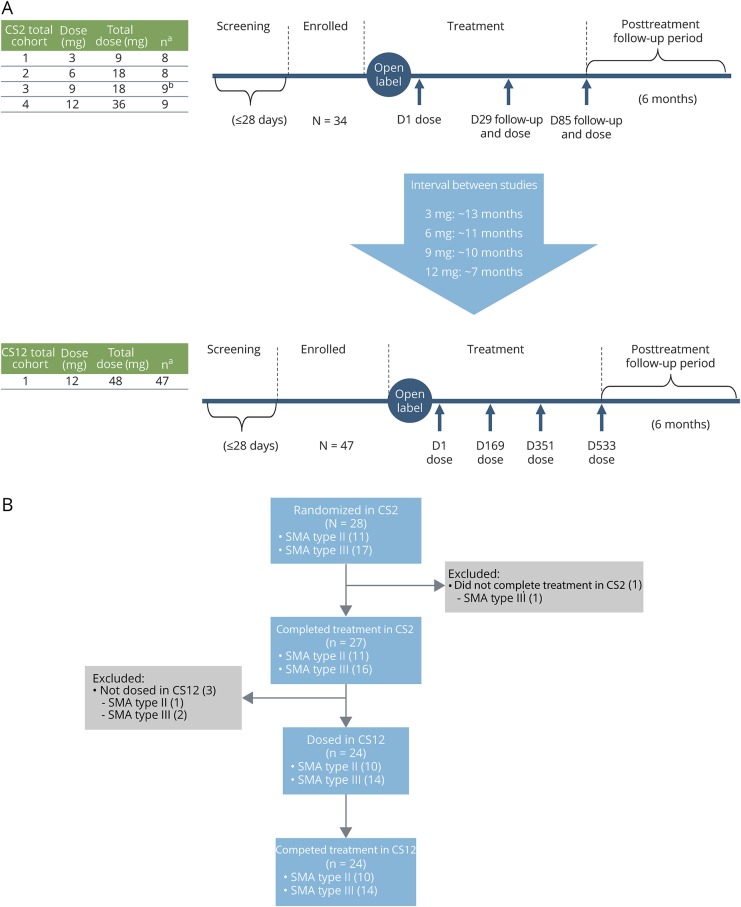
Figure 1. CS2 (phase 1b/2a open-label) and CS12 (extension) study designs and patient disposition.
(A) Study designs: aOverall enrollment. bPatients received treatment on days 1 and 85 only. (B) Patient disposition: The CS2-CS12 integrated efficacy analysis included 28 children from CS2 who received their first dose of nusinersen in CS2 and could have been treated in CS12. The total number of nusinersen doses administered in CS2 was 2 (cohort 3) or 3 (cohorts 1, 2, and 4), and the total number of nusinersen doses administered in CS12 was 4. D = day; SMA = spinal muscular atrophy.