Figure 1. Trial Design:
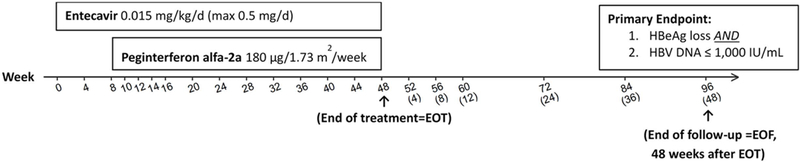
Entecavir 0.5 mg daily alone for 8 weeks followed by peginterferon alfa-2a 180 μg/week combined with entecavir for an additional 40 weeks. Endpoints were evaluated at the end of treatment (week 48) and 48 weeks after treatment completion (week 96). Weeks 52, 60, 72, and 96 correspond to 4, 12, 24, and 48 weeks after end of treatment, respectively, for those who discontinued treatment early.
