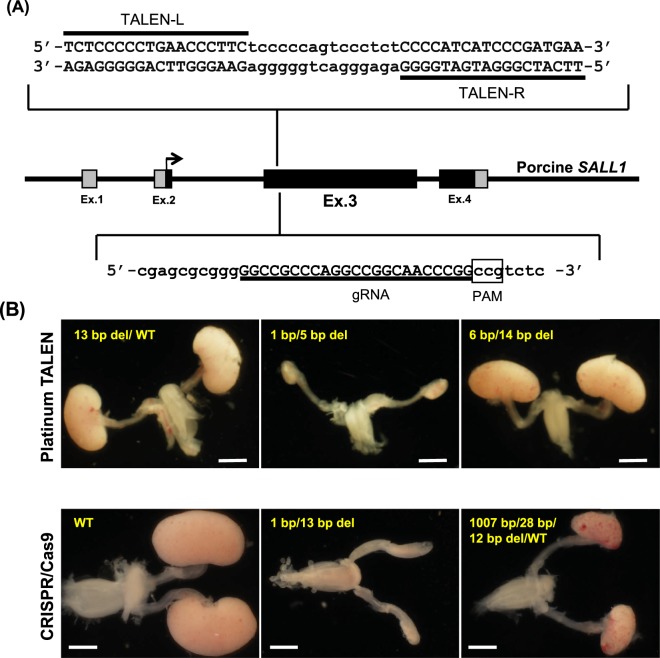
Figure 1.
Generation of porcine SALL1 gene knockout foetuses by genome editing. (A) Design of Platinum TALENs and CRISPR/Cas9 targeting the porcine SALL1 gene, which consists of 4 exons, similar to that of humans. The coding and untranslated regions are indicated by black and grey boxes, respectively. A pair of Platinum TALENs (top) and CRISPR/Cas9 (bottom) target the beginning of exon 3 in porcine SALL1. The protospacer adjacent motif (PAM) is a short specific sequence following the target DNA sequence that is essential for cleavage by Cas9 nuclease and is indicated with a white box. (B) Kidney phenotypes of the genome-edited foetuses with mutant SALL1. Foetuses developed from zygotes injected with Platinum TALENs (upper 3 panels) and CRISPR/Cas9 (lower 3 panels) were examined for nephrogenesis on days 36–37 and 39–40 of gestation, respectively. Kidneys with homozygous frameshift mutations (upper and lower middle) exhibited an anephrogenic phenotype. Foetuses harbouring WT SALL1 (13 bp del/WT, upper left) or SALL1 with a small in-frame mutation (6 bp/14 bp del, upper right) developed normal kidneys like those of a WT foetus (lower left). The kidneys of a foetus with complex mosaic mutations (1007 bp/28 bp/12 bp del/WT, lower right) showed mild hypoplasia. Scale bars: 2 mm.